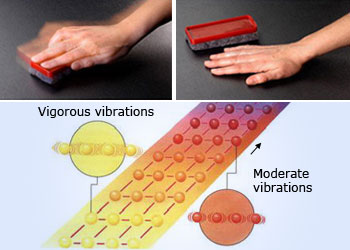
 Atoms movement due to thermal energy.
Vigorously rubbing the desktop with an eraser generates heat by transferring mechanical energy to thermal energy. You feel this as warmth. Molecules vibrate when heated. As the temperature rises, the particles have more kinetic energy and vibrates vigorously.
Atoms movement due to thermal energy.
Vigorously rubbing the desktop with an eraser generates heat by transferring mechanical energy to thermal energy. You feel this as warmth. Molecules vibrate when heated. As the temperature rises, the particles have more kinetic energy and vibrates vigorously.
Energy comes in various forms. Light, sound, mechanical energy, electrical energy, etc, are all different forms of energy. As we know, energy (and mass) can never be created or destroyed but it can be converted from one form into another.
Matter is composed of atoms and molecules. Atoms inside the molecules are never stationary. By virtue of their motion matter possesses kinetic energy. The average kinetic energy of the individual particles is directly related to a property you can sense: How hot something is. Whenever kinetic energy of its particles increases the substance becomes warmer.
Strike a coin with a hammer and it becomes warm because the hammer's blow causes the atoms in the metal to jostle faster. Rapidly compress air in a tyre pump and the air becomes warmer.
Stretch a rubber band and hold it on your lip; you will feel that it has become warmer. Heat and temperature are different quantities, although they are related to one another. Heat is the cause; temperature change is its effect.