 Semiconductor devices
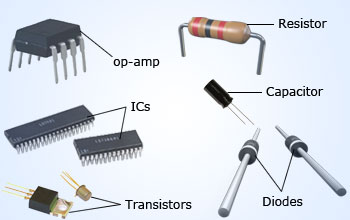
Electronic industries manufacture diodes, transistors, integrated circuits (ICs), etc, to develop different products for different applications. All these electronic devices are manufactured using semiconductor materials.
Semiconductor devices
Electronic industries manufacture diodes, transistors, integrated circuits (ICs), etc, to develop different products for different applications. All these electronic devices are manufactured using semiconductor materials.
 Click to watch video lesson
Click to watch video lesson
Electronics is the field of science and engineering which deals with semiconductor devices and their utilization. In today's world, electronics deals with diodes, transistors and their circuits. Microelectronics represents the Integrated Circuit (IC) technology, in which a circuit consists of millions of electronic components such as diodes, rectifiers, transistors, resistors and capacitors on a single piece of semiconductor chip, of area 100 mm2.
The present age is called the age of electronics as electronics have a wide range of applications such as amplification, rectification, communication, industrial automation, control of power generation, biomedical equipments, calculators, microprocessors, mobiles, computers, consumer electronics and photo-electricity, etc. Presently, electronic industries manufacture diodes, transistors, operational amplifiers (OPAMPs), integrated circuits (ICs) etc., to develop different products for different applications. All electronics devices starting from individual discrete devices such as diodes to very large scale integrated circuits are manufactured using semiconductors materials.