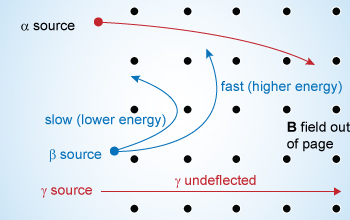
 Effect of a magnetic field on alpha, beta and gamma radiation
Alpha rays travel faster with higher energy and are less deflected when compared to ‘β’ rays. Gamma rays are not effected by the magnetic field,hence they go undeflected.
Effect of a magnetic field on alpha, beta and gamma radiation
Alpha rays travel faster with higher energy and are less deflected when compared to ‘β’ rays. Gamma rays are not effected by the magnetic field,hence they go undeflected.
A small hole is drilled in a Lead block ‘L’ and a piece of radium is placed in it. Radiations from radium escape only through the hole and others are absorbed by the wall. This arrangement is placed in an evacuated enclosure. The nature of radiations emitted by the radioactive source are studied by applying
i) electric field and ii) Magnetic field
Results for application of electric field
The first part of rays are called α–particles and they bends towards the negative plate. The deflection of α–particles towards the negative plate shows that they are positively charged. It has been found that α–particle is actually a helium nucleus (2He4).
The second part of rays bends towards the positive plate. This part constitutes β–particles. The deflection of β–particles towards the positive plate shows that they are negatively charged. It has been found that β–particles are electrons.
The third part of rays remains unaffected and goes straight without bending. This part constitutes γ–rays. This means that γ–rays are uncharged. It has been found that γ–rays are electromagnetic waves (photons) of very short wavelengths. These are emitted from the nucleus that is in the excited state. The α–particles bend towards the negative plate by a small amount showing that they are heavy positively charged particles.
On the other hand, β–particles bend towards the positive plate by a large amount showing that they are light negatively charged particles.
Results for application of magnetic field
When a uniform magnetic field is applied, perpendicular to the plane of the paper and directed into it then the beam splits into three. By applying Fleming's left hand rule, it can be seen that the ray which bends towards left is α–particle, the ray which bend towards right in a semi-circle is the β–particle and the ray which goes un–deflected is the γ–ray.
Using other experiments, it can be shown that the α–particles are ‘He’ atoms, β–particles are electrons and γ–rays are Electromagnetic radiations.