 Nuclear fission reaction
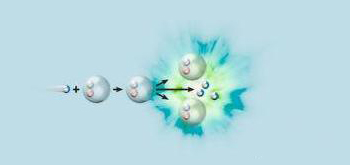
Nuclear fission reaction, computer artwork. At left is a neutron (blue) about to collide with an uranium-235 nucleus (grey). Upon collision the neutron combines with the nucleus to form uranium-236. This is a highly unstable element that spontaneously undergoes nuclear fission (splitting), producing a vast amount of energy (seen here as a blue and green explosion), the elements barium-141, krypton-92 and three neutrons. This is the reaction that occurred in the atomic bombs dropped by the Americans on the Japanese cities of Hiroshima and Nagasaki during World War II.
Nuclear fission reaction
Nuclear fission reaction, computer artwork. At left is a neutron (blue) about to collide with an uranium-235 nucleus (grey). Upon collision the neutron combines with the nucleus to form uranium-236. This is a highly unstable element that spontaneously undergoes nuclear fission (splitting), producing a vast amount of energy (seen here as a blue and green explosion), the elements barium-141, krypton-92 and three neutrons. This is the reaction that occurred in the atomic bombs dropped by the Americans on the Japanese cities of Hiroshima and Nagasaki during World War II.
 Click to watch video lesson
Click to watch video lesson
When a neutron collides with a nucleus, it may simply scatter of the nucleus, exchanging some energy of motion with it or get absorbed by the nucleus. When neutron is absorbed a new compound nucleus is formed.
Thus when a neutron is absorbed by U–235, the compound nucleus U–236 is formed with. The new compound nucleus will be unstable due to its excited state as it absorbs the binding energy of the neutron. It may simply emit a gamma ray and return to its ground state. It may expel a neutron, a proton or an alpha particle; or if the nucleus is very excited, it may even spill out more than one neutron or proton.
For a neutron absorbed by a heavy nucleus, there is another distinct mode of decay possible; the excited compound nucleus splits into roughly equal fragments–nuclei of intermediate mass number. This decay mode is nuclear fission–the process basic to nuclear reactors and atomic bombs.
The dynamics of nuclear fission can be understood in a simple way. Heavy nucleus is like a charged liquid drop with a near spherical shape. When it absorbs a neutron, some energy is added to it which gives rise to surface oscillations that stretch the drop further away from the spherical shape. If the added energy is sufficient, the original drop will split into two smaller drops which mean that the heavy nucleus has undergone fission.
Thus when a nucleus absorbs neutron, a variety of reactions is possible. An experimental measure of probability for each variety of reaction is called cross section of the reaction. The greater the cross section, the greater the likelihood of that reaction taking place.