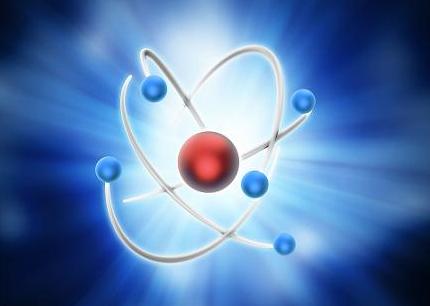
 Diagram of the structure of the atom
An atom consists of one or more electrons that whirl about the tiny, central nucleus. The nucleus consists of protons (red) & neutrons (blue). The number of electrically positive protons balance the number of electrically negative electrons to make the atom neutral overall.
Diagram of the structure of the atom
An atom consists of one or more electrons that whirl about the tiny, central nucleus. The nucleus consists of protons (red) & neutrons (blue). The number of electrically positive protons balance the number of electrically negative electrons to make the atom neutral overall.
The great Caltech Physicist Richard Feynman, in a series of lectures given for Caltech undergraduates observed that if scientific history had to be reduced to one important statement it would be "All things are made of atoms" –– little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.
Richard P. Feynman also shared some of his thoughts he had standing at the seashore. "There are the rushing waves... Mountains of molecules, each stupidly minding its own business, trillions apart, yet forming white surf in unison. Deep in the sea, all molecules repeat the pattern of one another till complex new ones are formed. They make others like themselves and a new dance starts. Growing in size and complexity – living things, masses of atoms, DNA, protein – dancing a pattern ever more intricate.
Out of the cradle onto dry land here it is standing: atoms with consciousness; matter with curiosity. Stands at the sea, wonders at wondering: I a universe of atoms an atom in the universe."