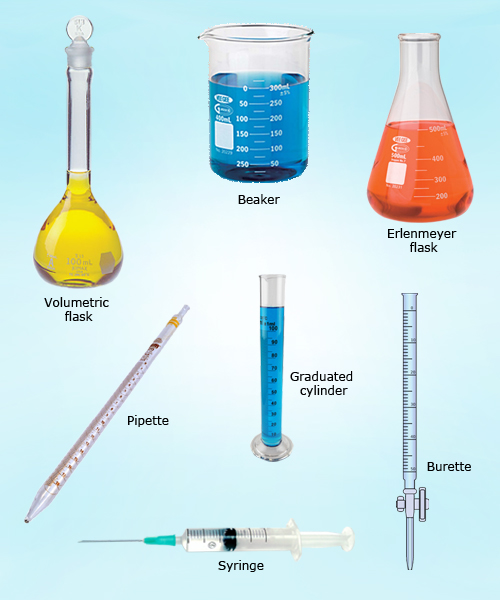
Volumetric analysis is also called as titrimetric analysis.
Titrate: Substance to be analyzed or to be determined.
Titrant: Reagent of know concentration
Titration: The process of determining the volume
Equivalence point: The point at which complete chemical reaction takes place and equivalent quantity of reagent is used
End Point: The point at which the indicator changes its color
Indicator: Auxiliary agents used to determine the end point of titration
Concentration: A defined quantity of substance in a defined volume of solution
Difference between a burette and a pipette :
Burette : Measures accurately the volume of a solution added. Readings can be taken to an accuracy of half a division, that is ± 0.05 cm3.
Pipette : Delivers an accurate volume of a solution. Often this is 25 cm3.