Rotation of sun is responsible for the variations in rates of decay – Purdue researchers
Stanford universities has found that the decay of radioactive isotopes fluctuates in sync with the rotation of the sun's core. The fluctuations appear to be very small but could lead to predictive tools for solar flares and may have an impact on medical radiation treatments.
This adds to evidence of swings in decay rates in response to solar activity and the distance between the Earth and the Sun that Purdue researchers Ephraim Fischbach, a professor of physics, and Jere Jenkins, a nuclear engineer, have been gathering for the last four years. The Purdue team previously reported observing a drop in the rate of decay that began a day and half before and peaked during the December 2006 solar flare and an annual fluctuation that appeared to be based on the Earth's orbit of, and changing distance from, the sun, Jenkins said.
“If the relationship between solar activity and decay rates proves to be true, it could lead to a method of predicting solar flares, which could help prevent damage to satellites and electric grids, as well as save the lives of astronauts in space“, Jenkins said. “Finding that the decay rates fluctuate in a pattern that matches known and theoretical solar frequencies is compelling evidence for a solar influence on decay rates“.
Jenkins and Fischbach collaborated with Peter Sturrock, a professor emeritus of applied physics at Stanford University and an expert on the inner workings of the sun, to examine data collected at Brookhaven National Laboratory on the rate of decay of the radioactive isotopes silicon–32 and chlorine-36. The team reported in the journal Astroparticle Physics that the decay rate for both isotopes varies in a 33-day recurring pattern, which they attribute to the rotation rate of the Sun's core.

Rotation of Sun influences age of artifact?
Dating of ancient artifacts may have influence of rotation of Sun. Influence of Sun's rotations in the determination of age of ancient artifacts is still a debatable issue which has no strong point of evidence at this moment. Much more research has to be done to have a close look into it.
In general, the fluctuations that Jenkins and Fischbach have found are around a tenth of a percent from what is expected, as they've examined available published data and taken some measurements themselves. The team has not yet examined isotopes used in medical radiation treatments or for dating of ancient artifacts.
“The fluctuations we're seeing are fractions of a percent and are not likely to radically alter any major anthropological findings“, Fischbach said. “One of our next steps is to look into the isotopes used medically to see if there are any variations that would lead to overdosing or underdosing in radiation treatments, but there is no cause for alarm at this point. What is key here is that what was thought to be a constant actually varies and we've discovered a periodic oscillation where there shouldn't be one“.
The Purdue team has ruled out the possibility of experimental error or an environmental influence on the detection systems that track the rate of decay as being responsible for the fluctuations and published a series of papers in the journals Astroparticle Physics, Nuclear Instruments and Methods in Physics Research, and Space Science Reviews.
Sturrock said it is an effect that no one yet understands and that if it is not neutrinos that are responsible, then perhaps there is an unknown particle interacting with the atoms.
“It would have to be something we don't know about – an unknown particle that is also emitted by the sun and has this effect – and that would be even more remarkable“, he said.

Solar Neutrino
Jenkins and Fischbach suggest that the changes in the decay rates are due to interactions with solar neutrinos, nearly weightless particles created by nuclear reactions within the sun's core that travel almost at the speed of light. It is estimated that about 60 billion solar neutrinos pass through a person's fingernail every second, but they are so weakly reactive that they pass right through the body without disturbing or changing anything, Jenkins said. “We haven't known the solar neutrino to interact significantly with anything, but it fits with the evidence we've gathered as the likely source of these fluctuations“, he said. “So, what we're suggesting is that something that can't interact with anything is changing something that can't be changed“.
Why some radioisotopes, such as U–238, found in nature, whereas others are not and must by synthesized? The key to answering this question is to realize that different nuclei undergo radioactive decay at different rates. Many radioisotopes decay essentially completely in a matter of seconds or less; obviously, we do not find such nuclei in nature. In contrast, uranium–238 decays very slowly; therefore, despite its instability, we can still observe this isotope in nature. An important characteristic of a radioisotope is its rate of radioactive decay.
Radioactive decay is a first–order kinetic process. This first-order process has a characteristic half–life, which is the time required for half of any given quantity of a substance to react. The rates of decay of nuclei are commonly discussed in terms of their half–lives.
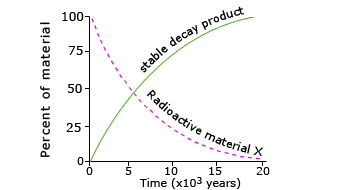
Decay curve
A radioactive decay curve showing the decay of material with time indicated with a dotted line. Standard line showing the formation of new stable product.
Each isotope has its own characteristic half–life. For example, the half-life of strontium–90 is 28.8 yr. If we started with 10.0 g of strontium–90, only 5.0 g of that isotope would remain after 28.8 yr, 2.5 g would remain after another 28.8 yr, and so on. Strontium–90 decays to yttrium–90, as shown in the below equation.
38 Sr90 → 39Y90 + -1e0
Half-lives as short as millionths of a second and as long as billions of years are known. One important feature of half-lives for nuclear decay is that they are unaffected by external conditions such as temperature, pressure, or state of chemical combination. Therefore, unlike toxic chemicals, radioactive atoms cannot be rendered harmless by chemical reaction or by any other practical treatment. At this point we can do nothing but allow these nuclei to lose radioactivity at their characteristic rates. In the meantime, of course, we must take precautions to isolate radio isotopes because of the damage radiation can cause.
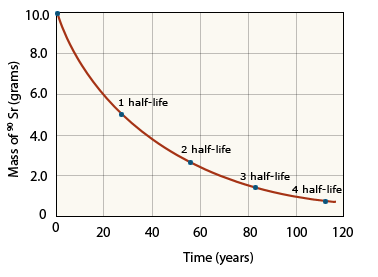
Decay of strontium –90
A radioactive decay curve showing the decay of strontium–90 with time indicating the significance of half life in finding the % of product reacted.