 Cathodic protection
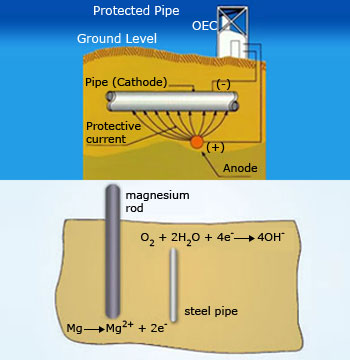
Cathodic protection (CP) is a technique used to control the corrosion
of a metal surface by making it the cathode of an electrochemical cell. The simplest method to apply CP is
by connecting the metal to be protected with another more easily corroded sacrificial metal to act as the
anode of the electrochemical cell. For structures where passive galvanic CP is not adequate, including long
pipelines, an external power source provides the current. Cathodic protection systems are used to protect a
wide range of metallic structures in various environments. Common applications are steel water or fuel
pipelines and storage tanks, steel pier pipes, ships and boats, offshore oil platforms and onshore oil well
casings and metal reinforcement bars in concrete buildings and structures.
Cathodic protection
Cathodic protection (CP) is a technique used to control the corrosion
of a metal surface by making it the cathode of an electrochemical cell. The simplest method to apply CP is
by connecting the metal to be protected with another more easily corroded sacrificial metal to act as the
anode of the electrochemical cell. For structures where passive galvanic CP is not adequate, including long
pipelines, an external power source provides the current. Cathodic protection systems are used to protect a
wide range of metallic structures in various environments. Common applications are steel water or fuel
pipelines and storage tanks, steel pier pipes, ships and boats, offshore oil platforms and onshore oil well
casings and metal reinforcement bars in concrete buildings and structures.
Prevention of corrosion
Corrosion of metals such as iron can be prevented by the following methods:- Cathodic protection
- Using a sacrificial layer such as Zn, known as galvanizing
- Electroplating
- Painting
- Alloying
 Mg2+(aq) + 2e−
Mg2+(aq) + 2e−
 4OH−(aq)
4OH−(aq)
Galvanization
A protective water insoluble oxidized coat is the principle underlying a process called galvanization. Zinc has a slightly greater tendency to oxidize than iron. For this reason, many iron articles such as nails are galvanized by coating them with a thin layer of zinc. The zinc oxidizes to zinc oxide, an inert, insoluble substance that protects the inner iron from rusting. The galvanized nail is protected from rusting by the sacrificial oxidation of zinc.
 Stainless steel
Stainless steel, also known as inox (meaning "inoxydable" in French) is
defined as a steel alloy with a minimum of 10.5% to 11% chromium content by mass.
Stainless steel
Stainless steel, also known as inox (meaning "inoxydable" in French) is
defined as a steel alloy with a minimum of 10.5% to 11% chromium content by mass.

Yet another way to protect iron and other metals from oxidation is to coat them with a corrosion–resistant metal, such as chromium, platinum, or gold. Electroplating is the operation of coating one metal with another by electrolysis. The object to be electroplated is connected to a negative battery terminal and then submerged in a solution containing ions of the metal to be used as the coating.
The positive terminal of the battery is connected to an electrode made of the coating metal. The circuit is completed when this electrode is submerged in the solution. Dissolved metal ions are attracted to the negatively charged object, where they pick up electrons and are deposited as metal atoms. The ions in the solution are replenished by the forced oxidation of the coating metal at the positive electrode. Iron is generally electroplated with less oxidizing metals like chromium or tin.
Painting a corroding surface with an oxidizing element, so that the underlying metal is protected, is routinely done. The paint generally contains lead oxide, calcium oxide and other compounds – already oxidized compounds.
Another technique to avoid corrosion so that the life of the metal is made longer is to alloy that metal with some elements so that the corrosion is harnessed. Stainless steel, which is an alloy of iron, carbon, nickel, etc., (there are various types of steel, which vary in their elemental compositions) does not rust at all.