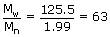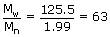Poly Dispersity Index (PDI)
Macromolecules are the "giant" molecules (polymers) formed when small
organic molecules (monomers) are joined in a biological medium. Example: protein. Due to
joining of many monomer units, the molecule has high molecular weight and high
viscosity.
Macromolecules have a molar mass distribution due to the simplest homopolymers made of homologue chains with a different number of repeating units. They exhibit average values of two types.
i. Number average molar mass [Mn]
ii. Weight average molar mass [Mw]
The ratio of these average values give the Poly Dispersity Index (PDI) = Mw / Mn
Mn and Mw depend on the polymer chains of definite
molar mass.
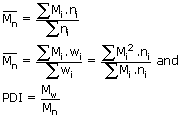
Calculating PDI
Q1
If the 'number average molecular weight' and 'weight average molecular weight' of a polymer are 40,000
and 60,000 respectively, then calculate the polydispersity index of the polymer. Based on the value,
predict the nature of polymer.
Sol:
Given, Mw 60,000 and Mn = 40,000.
Therefore, PDI = Mw/Mn = 60,000/40,000 = 1.5 (>1).
The polymer with the value (>1) have monomer units arranged in chains of different length.
Mw/Mn>1⇒ Mw>Mn ⇒ most probably it is a
natural polymer.
When the PDI value is one, it indicates mono dispersity or given polymer has monomers
arranged in chains of equal length.
Q2
Why does a macromolecule have several molar masses and what do these indicate ?
Sol:
Macromolecule (with exception of natural proteins
and DNA) have molar mass distribution (MMD). This means that the even simplest homopolymers
consists of homologue chains with a different number of repeated units. Therefore, unlike
low molecular-weight components, macromolecules do not exhibit one definite molar mass. They
are normally described with statistical based molar mass average values such as the molar
mass averages of the number (Mn) or weight (Mw) and the polydispersity
index (PDI)
Q3
Why are these averages important ?
Sol:
Mn and Mware calculated by averaging the number or the weight of
polymer chains with a defined molar mass
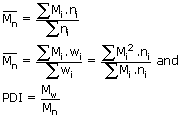
calculating Mw, Mn and PDI
Consider two polymers names A and B of each 1000 kg weight
| Composition A | Mn | M w |
|---|---|---|
|
500 stones with 1 kg = 500 kg
2 stones with 250 kg = 500 kg
Total = 1000 kgs
|
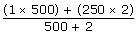 =1.99
|
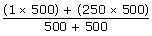 =125.5
|
|
composition B
400 stones with 1 kg = 400 kg
100 stones with 6 kg = 500 kg
Total = 1000 kgs
|
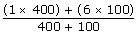 =2.00
|
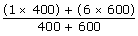 =125.5
|
where (Mn) = number average molecular weight
(Mw) = weight average molecular weight
The polydispersity index (PDI) for A and B is therefore
PDI =