An oxy acid is any acid containing oxygen. Most covalent nonmetallic oxides react with water to form acidic oxides; that is, they react with water to form oxy-acids that yield hydronium ions (H3O+) in solution. Exceptions are CO, N2O, and NO.
Following are the oxyacids of Nitrogen:
1.Hyponitrous acid: HNO (OR) H2N2O2
2.Nitrous acid: (HNO2)
3.Nitric acid: (HNO3)
1.Hyponitrous acid : HNO (OR) H2N2O2 :
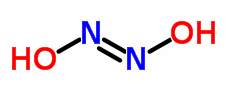
In aqueous solution, it is a weak acid and decomposes to nitrous oxide (N2O) and water. Since this reaction is not reversible, N2O should not be considered as the anhydride of H2N2O2
H2N2O2 → N2O + H2O.
Nitrous acid (HNO2) :
1. Methods of preparation :
In the laboratory it is prepared by the addition of ice cold dilute acid to Barium nitrite
Ba(NO3)2 + H2SO4 → BaSO4 + 2HNO2
Its solution is slightly bluish in colour due to the presence N2O3.
In the gas phase nitrous acid can be made by the following reaction:
NO + NO2 + H2O → 2 ⇌ 2HNO2
On standing it undergoes auto oxidation-reduction
3HNO2 → HNO3 + 2NO + H2O
In this reaction:
In HNO2 → HNO3 (O.S of 'N' changes from + 3 to 5)
In HNO2 → NO (O.S of 'N' changes from + 3 to + 2)
i.e HNO2 as oxidant changes to NO and as reductant changes to HNO3
With oxidizing agents stronger than HNO2 like KMnO4, K2Cr2O7, (Br2 + H2O or H2O2 solutions) HNO2 functions as reductant.
Where as with weaker oxidants i.e reducing agents like H2S, SO2 or Sn+2 solutions HNO2 functions as oxidant.
At low temperatures HNO2 reacts with aromatic primary amines and gives diazonium compounds.
Diazonium compounds can be converted into different substituted aromatic compounds.
2. Structure of (HNO2):
HNO2 exists in two tautomeric forms.
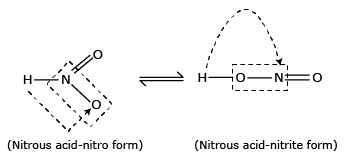
HNO2 is a weak acid and its salts are known as nitrites
Ex : Sodium Nitrite NaNO2
It is obtained by dissolving N2O3 in water.
It is an unstable and weak acid.
It acts as an oxidizing and reducing agent.