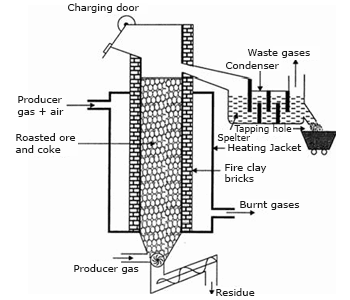
Extraction of Zinc
The important ore of Zinc is Zinc blende (ZnS). So it is extracted from a zinc blend by the carbon
reduction process. The following are the steps involved in the extraction process of zinc.
a. Crushing and Pulverization:
The big lumps of ore are first crushed by a jaw crusher and powdered in ball mills.
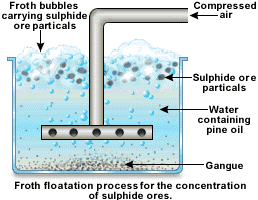
b. Concentration:
The crushed and pulverized ore is concentrated by the froth flotation process. This process is used for the concentration of sulphide ore. The powered ore is suspended in water and after adding pine, it is stirred by passing the compressed air. The particles of the sulphide ore which are wetted by the oil come to the surface along the froth (fizzy substance) which is taken out and the gangue particles remains at the bottom and are then removed.
c. Roasting:
The concentrated ore is roasted in the excess air at a temperature of 900°C in the
reverberatoryfurnace. Here, the ore reacts with oxygen to form Zinc Sulphide (ZnS) and Sulphur
dioxide (SO2) which is given by the reaction below.
2ZnS + 3O2 → 2ZnO + SO2
Reverberatory furnace
During this process, some more of the ZnS is also converted into zinc sulphate.
ZnS + 2O2 → ZnSO4
Above 900°C, this zinc sulphate decomposes to give Zinc Oxide.
ZnSO4- → 2ZnO + SO2 + O2
In this roasting process, the volatile impurities are also removed as their oxides.
C + O2 → CO2
S + O2 → SO2
P4 + 5O2 → 2P2O5
2As + 3O2 → 2As2O3