Aldehydes and Ketones undergo the following reactions:
- Nucleophillic addition reactions,
- Nucleophillic addition – elimination reactions,
- α – substituted reactions,and
- carbonyl –carbonyl reactions.
The most common reactions are nucleophilic addition reactions, which lead to the formation of alcohols, alkenes, diols, cyanohydrins (RCH(OH)CN), and imines( R2CNR).
| Reaction name | Substrate | Comment |
|---|---|---|
| Ozonolysis | alkene | ozonolysis of non–fully–substituted alkenes yield aldehydes upon reductive work–up. |
| Organic reduction | ester | Reduction of an ester with diisobutylaluminum hydride (DIBAL–H) or sodium aluminum hydride |
| Rosenmund reaction | acid chloride | or using lithium tri–t–butoxyaluminum hydride (LiAlH(OtBu)3). |
| Wittig reaction | ketone | reagent methoxymethylenetriphenylphosphine in a modified Wittig reaction. |
| Formylation reactions | nucleophilic arenes | various reactions for example the Vilsmeier–Haack reaction |
| Nef reaction | Nitro compound | |
| Zincke reaction | pyridines | Zincke aldehydes form in a variation |
| Stephen aldehyde synthesis | nitriles | reagents tin(II) chloride and hydrochloric acid. |
| Meyers synthesis | oxazine | oxazine hydrolysis |
| McFadyen–Stevens reaction | hydrazide | is a base–catalyzed thermal decomposition of acylsulfonylhydrazides |
The C=O bond of aldehydes and ketones reacts with nucleophiles (such as H–, an organometallic reagent, or CN–)in nucleophillic reactions. Nucleophiles add more rapidly to aldehydes than ketones because of steric and electronic effects. The reactions of nucleophilic groups containing oxygen,sulfur or nitrogen with C = O bond are reversible and are catalyzed by an acid.
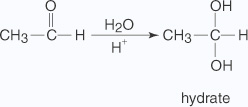
- Addition of water
The addition of water to an aldehyde results in the formation of a hydrate.The formation of a hydrate proceeds via a nucleophilic addition mechanism.
- Water, acting as a nucleophile, is attracted to the partially positive carbon of
the carbonyl group,
generating an oxonium ion.
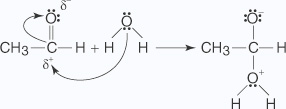
- The oxonium ion liberates a hydrogen ion that is picked up by the oxygen anion in
an acid–base reaction.
 Small amounts of acids and bases catalyze this reaction. This occurs because the addition of acid causes a protonation of the oxygen of the carbonyl group, leading to the formation of a full positive charge on the carbonyl carbon, making the carbon a good nucleus.Adding hydroxyl ions changes the nucleophile from water (a weak nucleophile) to a hydroxide ion (a strong nucleophile). Ketones usually do not form stable hydrates.
Small amounts of acids and bases catalyze this reaction. This occurs because the addition of acid causes a protonation of the oxygen of the carbonyl group, leading to the formation of a full positive charge on the carbonyl carbon, making the carbon a good nucleus.Adding hydroxyl ions changes the nucleophile from water (a weak nucleophile) to a hydroxide ion (a strong nucleophile). Ketones usually do not form stable hydrates. - Addition of Hydrogen cyanide
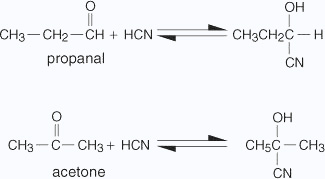
Aldehydes and ketones react with hydrogen cyanide (HCN) to yield cyanohydrins. This reaction occurs very slowly with pure HCN. Therefore, it is catalyzed by a base and the generated cyanide ion (CN–) being a stronger nucleophile readily adds to carbonyl compounds to yield corresponding cyanohydrin.Cyanohydrins are useful synthetic intermediates.The addition of hydrogen cyanide to a carbonyl group of an aldehyde or most ketones produces a cyanohydrin. Sterically hindered ketones, however, don't undergo this reaction.The mechanism for the addition of hydrogen cyanide is a straightforward nucleophilic addition across the carbonyl carbony oxygen bond.
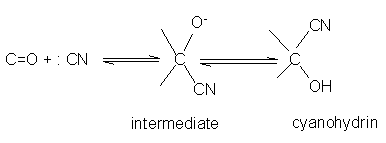
- Addition of sodium hydrogen sulphite(NaHSO3) Sodium
hydrogensulphite adds to aldehydes and ketones to form the addition products.
The position of the equilibrium lies largely to the right hand side for most aldehydes and to the left for most ketones due to steric reasons.
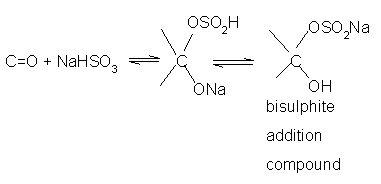 The hydrogensulphite addition compound is water soluble and can be converted back to the original carbonyl compound by treating it with dilute mineral acid or alkali.Therefore, these are useful for separation and purification of aldehydes.
The hydrogensulphite addition compound is water soluble and can be converted back to the original carbonyl compound by treating it with dilute mineral acid or alkali.Therefore, these are useful for separation and purification of aldehydes. - Addition of alcohol Reactions of aldehydes with alcohols produce either hemiacetals (a functional group consisting of one – OH group and one – OR group bonded to the same carbon) or acetals (a functional group consisting of two &ndashOR groups bonded to the same carbon), depending upon conditions. Mixing the two reactants together produces the hemiacetal. Mixing the two reactants with hydrochloric acid produces an acetal.
EXAMPLE:
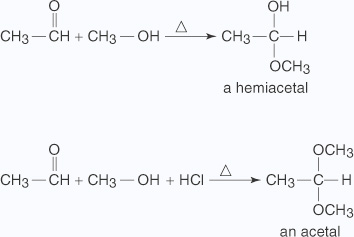
A nucleophilic substitution of an OH group for the double bond of the carbonyl group forms the hemiacetal through the following mechanism:
- An unshared electron pair on the alcohol's oxygen atom attacks the carbonyl
group.
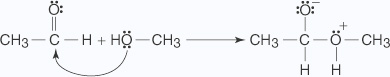
- The loss of a hydrogen ion to the oxygen anion stabilizes the oxonium ion formed in
Step
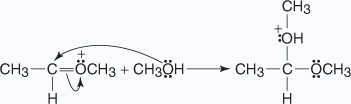
The addition of acid to the hemiacetal creates an acetal through the following mechanism:
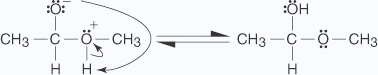
- The proton produced by the dissociation of hydrochloric acid protonates the alcohol
molecule in an acid–base reaction.
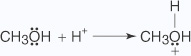
- An unshared electron pair from the hydroxyl oxygen of the hemiacetal removes a
proton from the protonated alcohol.
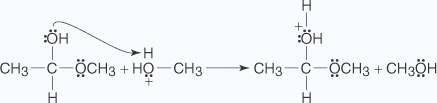
- The oxonium ion is lost from the hemiacetal as a molecule of water.
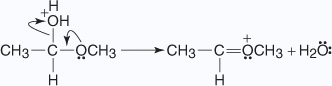
- A second molecule of alcohol attacks the carbonyl carbon that is forming the
protonated acetal.
- The oxonium ion loses a proton to an alcohol molecule, liberating the acetal.
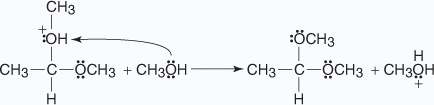
HydrolyzedStability of Acetals
Acetal formation reactions are reversible under acidic conditions but not under alkaline conditions. This characteristic makes an acetal an ideal protecting group for aldehyde molecules that must undergo further reactions. A protecting group is a group that is introduced into a molecule to prevent the reaction of a sensitive group while a reaction is carried out at some other site in the molecule. The protecting group must have the ability to easily react back to the original group from which it was formed. An example is the protection of an aldehyde group in a molecule so that an ester group can be reduced to an alcohol.
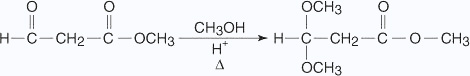
In the previous reaction, the aldehyde group is converted into an acetal group, thus preventing reaction at this site when further reactions are run on the rest of the molecule.
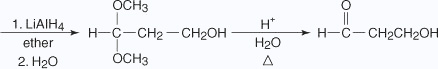
Notice in the previous reaction that the ketone carbonyl group has been reduced to an alcohol by reaction with LiAlH4. The protected aldehyde group has not been reduced. Hydrolysis of the reduction product recreates the original aldehyde group in the final product.
- Addition of ylides(The Wittig reaction)
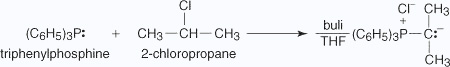
The reaction of aldehydes or ketones with phosphorus ylides produces alkenes of unambiguous double–bond locations. Phosphorous ylides are prepared by reacting a phosphine with an alkyl halide, followed by treatment with a base. Ylides have positive and negative charges on adjacent atoms.The following illustration shows the preparation of 2–methylbutene by a Wittig reaction.
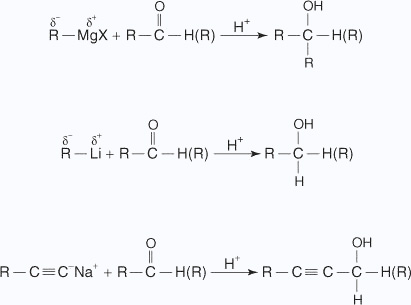
- Addition of organometallic reagents
Grignard reagents, organolithium compounds, and sodium alkynides react with formaldehyde to produce primary alcohols, all other aldehydes produce secondary alcohols, and ketones to produce tertiary alcohols.