Common names for ketones can be derived by naming the two alkyl or aryl groups bonded to the carbonyl group as separate words followed by the word ketone.
Acetone
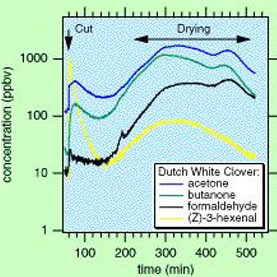
Green clover leafs release acetone into the atmosphere which is stable in the lower atmosphere, but undergoes photolysis, contributing to the oxidative balance of the Troposphere–stratosphere boundary.

Acetophenone
 Apricot tree
Apricot tree
 Synthetic musk
Synthetic musk
Benzophenone
Ethyl isopropyl ketone
Diethyl ketone


Aldehydes

 Green apple
Green apple
 Bruised apples
Bruised apples

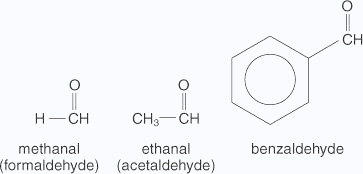
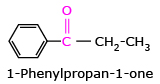
The common names of ketones are derived by naming two alkyl or aryl groups bonded to the carbonyl group. The locations of substituents are indicated by Greek letters, α, α', β, β' and so on beginning with the carbon atoms next to the carbonyl group, indicated as αα'. Some ketones have historical common names, the simplest dimethyl ketone is called acetone. Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
The IUPAC names of open chain aliphatic aldehydes and ketones are derived from the names of the corresponding alkanes by replacing the ending e with al and one respectively. In case of aldehydes the longest carbon chain is numbered starting from the carbon of the aldehyde group while in case of ketones the numbering begins from the end nearer to the carbonyl group.
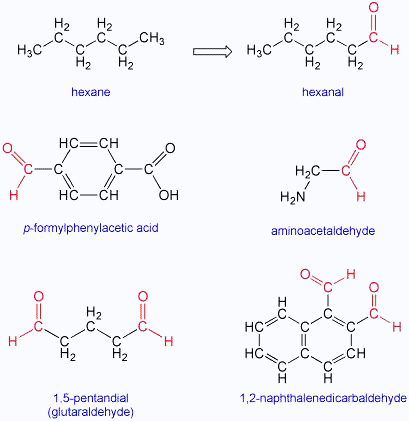
Aliphatic aldehydes with numbering
Aliphatic ketones
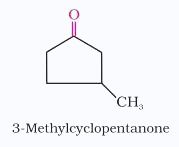
The substituents are prefixed in alphabetical order along with numerals indicating their positions in the carbon chain. The same applies to cyclic ketones, where the carbonyl carbon is numbered one.