Aldehydes and Ketones
An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. This makes the aldehydes very easy to oxidize.
Example:
ethanal, CH3CHO
It is very easily
oxidized to either ethanoic acid, CH3COOH, or ethanoate ions,
CH3COO–.
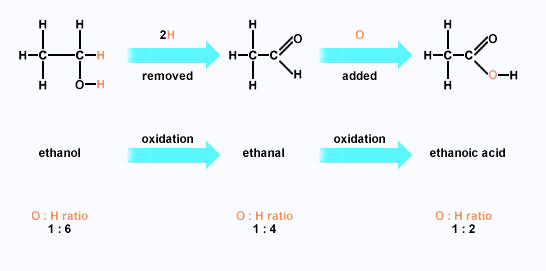
Ketones don't have that hydrogen atom and are resistant to oxidation.
They are only oxidized by powerful oxidizing agents which have the ability
to break carbon-carbon bonds.