
Taj Mahal is located at Agra, India. Many industries in the surrounding areas release large quantities of the oxides of sulfur and nitrogen. The refinery set up at Mathura became another large source of sulfur oxides. The acidic gases such as SO2, SO3 and NO2 react with water vapor present in the atmosphere or with rain water to give sulfuric and nitric acids. These acids react with the marble of the Taj Mahal to form calcium sulphate and calcium nitrate.
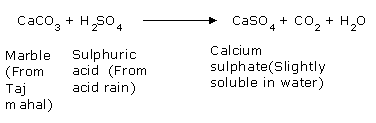
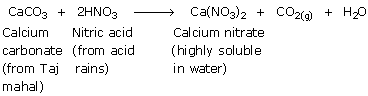
The salts calcium sulphate (CaSO4) and calcium nitrate (Ca(NO3)2) dissolve in water. As a result, marble of the Taj Mahal would get corroded and lose its lustre due to its reaction with the acidic gases present in the atmosphere or with the acid rain. These reactions are very slow and it takes a long time to damage such monuments/buildings. To constrain the damage to the Taj Mahal caused by the oxides of sulfur and nitrogen, the government of India announced an action plan in 1995. According to this plan,
- The air in the Taj Trapezium – the area including the towns of Agra, Firozabad, Mathura and Bharatpur should be pollution free.
- About 2000 industries located in this area should stop using coal or kerosene/furnace oil and switch over to either natural gas or LPG.
- The people living in this area should be encouraged to use LPG in place of coal/wood or kerosene.
- The vehicles running through this area including the highways should be encouraged to use low–sulfur diesel.