Nitrogen derivatives
Methods of preparation of amines:(IPE)
1. Reduction of nitriles:Nitriles can be reduced using
LiAlH4 or H2/Ni or Na(Hg)/C2H5OH, produce primary amines.
The amine produced has one carbon more than the alkyl group in the nitrile. Ex:Methyl cyanide
produces ethyl amine.
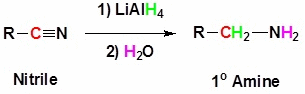
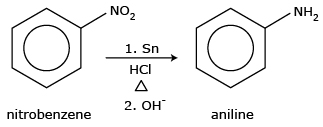
2. Reduction of nitro compounds:Reduction of nitro
compounds:These compounds can be reduced by catalytic hydrogenation using molecular hydrogen or
by chemical reduction using metal and acid. Aromatic amines are normally prepared by reduction of the
corresponding aromatic nitro compound.
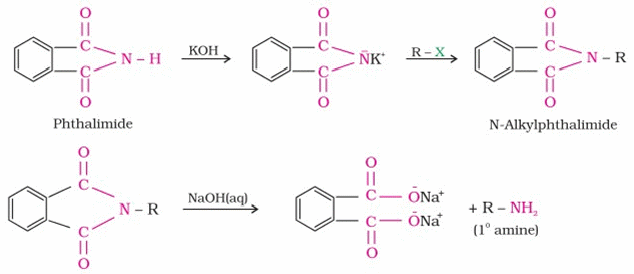
3. Gabriel synthesis :This is a method for preparing
aliphatic primary amines. In this method Phthalimide first reacts with alcoholic KOH to form
potassium salt of phthalimide, which on heating with an alkyl halide followed by alkaline
hydrolysis produces a primary amine as shown. This method cannot be used to prepare aromatic
primary amines since the anion of the phthalimide cannot react with aryl halides.
4. Hoffmann bromamide reaction:This is another method
for the preparation of primary amines. An amide is treated with bromine in an aqueious or
alcoholic solution of NaOH to produce the primary amine as shown.
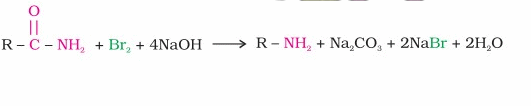
There is migration of an alkyl or aryl group from the carbonyl carbon of the amide to the nitrogen
atom. This reaction is also called the bromamide degradation ,since the product amine has one carbon
less than the parent amide.