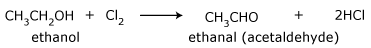
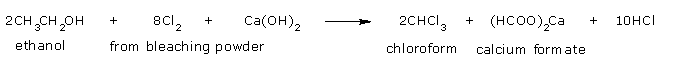
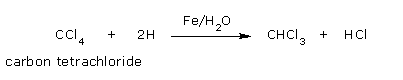Chloroform from ethanol
- Oxidation of ethyl alcohol by chlorine
- Formation of chloral by the action of chlorine on acetaldehyde so formed.
- Hydrolysis of chloral to chloroform by calcium hydroxide or alkali (NaOH).
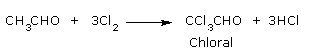
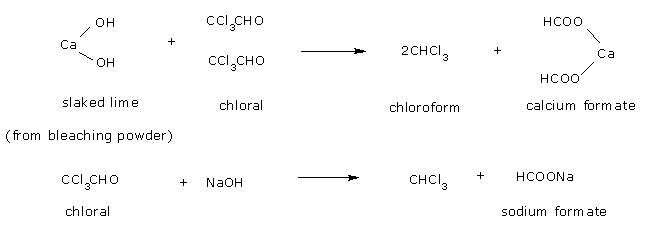
The net
reaction when bleaching powder is used is,

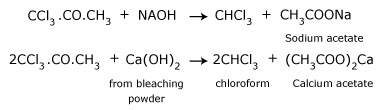
From acetone (from propanone):
When acetone is heated with bleaching powder, or with chlorine and alkali, the following reactions take place.
- Reaction of chlorine with acetone producing trichloroacetone.
- Trichloroacetone is decomposed by either calcium hydroxide or an alkali, to give chloroform.
- The net reaction when bleaching powder is used is,
- By partial reduction of carbon tetrachloride
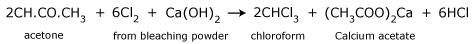
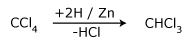
Zinc and hydrochloric acid is taken and shaken at a slightly elevated temperature.
Manufacture of Chloroform
Chloroform is generally manufactured by the following methods.
From methane: Chloroform can be manufactured by chlorinating methane. A mixture
of
CH4 :
Cl2 : N2 in the ratio 8 : 3 : 80 is passed over a partially
reduced sample of
cupric chloride. Chloroform is recovered by distillation.
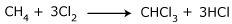
From carbon tetrachloride: Chloroform can also be prepared on a large scale by
partial
reduction of carbon tetrachloride using iron filings and water.