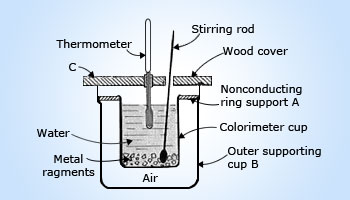
 Diagram of calorimeter
Diagram of calorimeter
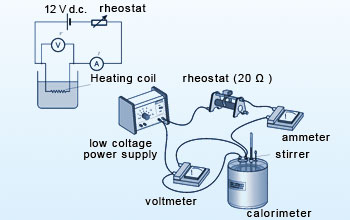 Experimental setup to measure specific heat
Experimental setup to measure specific heat
Calorimetry is an experimental technique for the quantitative measurement of heat exchange. We know that heat flows from a hot body to a cold body. When the two bodies are isolated from their surroundings, the amount of heat lost by the hot body must be equal to the amount of heat gained by the cold body. The same principle is used in calorimetry.
As heat is energy in transit, this principle is really just conservation of energy. Calorimetry, dealing entirely with one conserved quantity, is in many ways the simplest of all physical theories. One of the important uses of calorimetry is the determination of specific heats of unknown materials. A calorimeter is a device used to measure the amount of heat exchange. A simple calorimeter is shown in figure.
It is important that the calorimeter be well insulated so that almost no heat is exchanged with the surroundings. The insulated lid, jacket and air space are central to preventing heat loss to the surroundings. In the technique known as the "method of mixtures", a sample of the substance is heated to a high temperature which is accurately measured. The substance is then placed quickly in the calorimeter containing known mass of water at known temperature. The contents are stirred constantly until the mixture attains the final common temperature. The heat lost by the substance will be gained by the water and the calorimeter.
- Let
- m1 = Mass of water in the calorimeter
- m2 = Mass of the substance
- mC = Water equivalent of the calorimeter
- T1 = Initial temperature of water and calorimeter
- T2 = Initial temperature of the substance
- T = Final temperature of the mixture
- cS = Specific heat of the substance
- cW = Specific heat of water
- We already know that the heat lost or gained by a body is given by Q = mcΔT
- Therefore, heat lost by the substance is = m2cS(T2 ‐ T)
- Heat gained by water and calorimeter = m1cW(T ‐ T1) + mCcC(T ‐ T1)
- According to the law of heat exchange, Heat lost = Heat gained
- Or m2cS(T2 ‐ T) = m1cW(T ‐ T1) + mCcC(T ‐ T1)
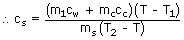
Since the values of the other variables are known, the specific heat of the substance can be calculated.