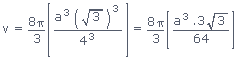
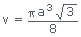

- 4
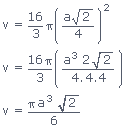

In the body centered cubic structure the number of atoms per unit cell are 2
Volume of 2 atoms (spherical) = 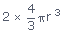
Radius of atom in BCC is r = 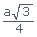
Volume occupied by atoms per unit cell (v) = 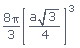
Volume occupied by atoms per unit cell (v)
Volume of unit cell for a cubic system (V) = a3
Therefore, we can say that 68% volume of the unit cell of BCC is occupied by atoms and the remaining 32% is vacant.
Since the packing density is greater than a simple cube, it has a tightly packed structure when compared to a simple cube.
Face Centered Cubic (FCC):
In the case of face-centered cubic structures, the number of atoms per unit cell is 4:
Volume of 4 atoms = 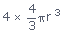
We know that the radius of the atom in FCC, r = 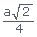
Volume occupied by the atoms per unit cell (v)
Volume of unit cell for a cubic system (V) = a3
Hence, the packing factor is 0.74, which shows that there is much more close packing present in FCC than BCC.
Here, 74% volume of the unit cell of a simple cube is occupied by atoms, and the remaining 26% volume is vacant.