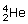 nucleus whose
mass is 6.6447 × 10− 27 kg. For this nucleus,
find
nucleus whose
mass is 6.6447 × 10− 27 kg. For this nucleus,
find(a) The helium nucleus contains Z = 2 protons and N = 4 − 2 = 2 neutrons. To obtain the mass defect Δm, we first determine the sum of the individual masses of the separated protons and neutrons.
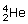 nucleus.
nucleus.The sum of the individual masses of the nucleons is 2(1.6726 × 10− 27 kg) + 2(1.6749 × 10− 27 kg) = 6.6950 × 10− 27 kg
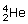 nucleus,
and the mass defect is
nucleus,
and the mass defect isΔm = 6.950 × 10− 27 kg − 6.6447 × 10− 27 kg = 0.0503 × 10− 27 kg
Usually, binding energies are expressed in energy units of electron volts instead of joules (1 eV = 1.60 × 10− 19 J):
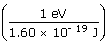 = 2.83
× 107 eV = 28.3 MeV
= 2.83
× 107 eV = 28.3 MeVn this result, one million electron volts is denoted by the unit MeV. The value of 28.3 MeV is more than two million times greater than the energy required to remove an orbital electron from an atom.