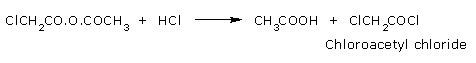Reactions of Acyl halide
- Ammonolysis:
- Alcoholysis:
- Reaction with salts of acid:
- Reaction with Ethers:
- Reaction with amines:
- Reaction with Grignard reagents:
- Reaction with alkoxides:
They react with ammonia and amines to give amides. The reaction involving cleavage of a bond with
ammonia is called ammonolysis.
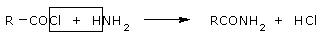
The reaction involving cleavage of a bond with Primary amine.
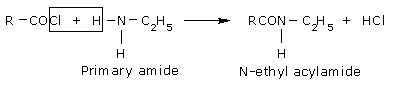
The reaction involving cleavage of a bond with Secondary amine.
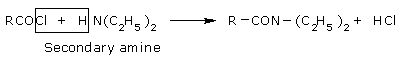
Tertiary amines do not react with Acetyl chlorides.
Acetyl chlorides react with alcohols and phenols to give esters. The cleavage of a bond with
alcohol is called alcoholysis.
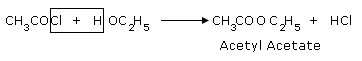
Acetyl chlorides react with salts of carboxylic to form acid anhydrides.
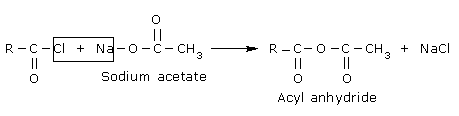
In these reactions, an acyl group is transferred to the nucleophiles. These reactions are known as
acylation reactions. Acylation is generally done in the presence of a base in order to neutralise
the HX formed. Aliphatic acyl chloride are very reactive acylating agents. Aromatic acyl chlorides
like benzoyl chloride are less reactive.
Acyl chlorides react with ethers to form alkyl chlorides and alkyl acetate in presence of zinc
chloride.
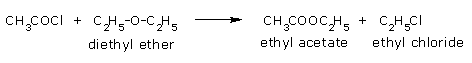
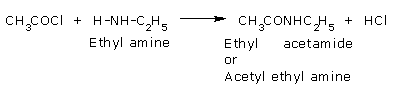
Acyl chlorides react with amines to form acetyl derivatives.
Acyl chlorides react with Grignard reagents to form Ketones.
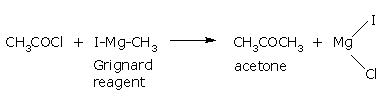
Acyl chlorides react with alkoxides to form esters.
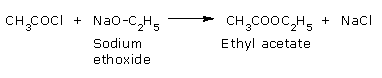
Nomenclature
Symmetrical anhydrides of unsubstituted carboxylic acids are derived from the names of the carboxylic acids by replacing the word acid with anhydride.
Symmetrical anhydrides of substituted carboxylic acids are named by adding the prefix bis to the name
to indicate that two identical acyl groups are present.
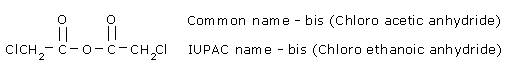
Unsymmetrical anhydrides are named by writing the names of the two acids alphabetically before the word anhydride.
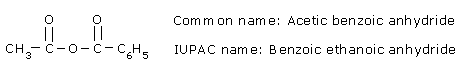
Preparation methods
- By Carbonylation of Methyl Acetate:
- By addition of Acetic acid to Ketene:
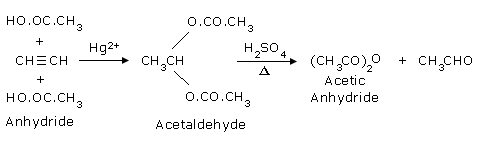
- By passing Acetylene into glacial acetic acid in presence of mercury salts as catalyst:
Acetic anhydride is produced by carbonylation of methyl acetate.
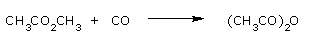
This process involves the conversion of methyl acetate to methyl iodide and an acetate salt.
Carbonylation of the methyl iodide in turn affords acetyl iodide, which reacts with acetate salts or
acetic acid to give the product. Rhodium iodide and lithium iodide are employed as catalysts.
Because acetic anhydride is not stable in water, the conversion is conducted under anhydrous
conditions.
To a decreasing extent, acetic anhydride is also prepared by the reaction of
ethenone (ketene) with acetic acid at
45–55°C and low pressure (0.05 – 0.2bar).
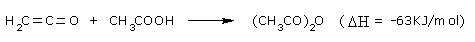
Ketene is generated by dehydrating acetic acid at 700–750°C in the
presence of triethyl phosphate as a catalyst
or (in Switzerland and the CIS) by the thermolysis of acetone at 600–700°C
in the presence of carbon disulfide as a catalyst.
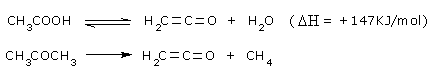
The route from acetic acid to acetic anhydride via ketene was developed by Wacker
Chemie in 1922,
when the demand for acetic anhydride increased due to the production of cellulose
acetate.
Ethylidene acetate so produced is distilled with conc.H2SO4 or
ZnCl2 as a
catalyst to get acetic anhydride.
Chemical reactions
Hydrolysis:
They are hydrolyzed by water slowly in cold and rapidly when heated to form Acetic acid.
Alcoholysis:
They form esters with alcohols and phenols.
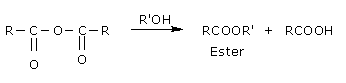
Reaction with ammonia:
They form amides with ammonia and amines.
With ammonia
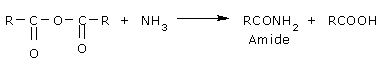
Friedel Craft's acylation reaction:
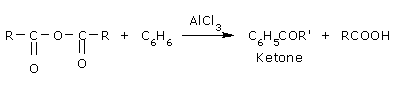
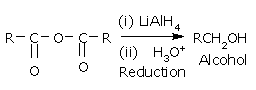
Anhydrides are on the whole less reactive than acyl chlorides.
Reaction with Hydrogen chloride:
Acetic anhydride react with Hydrogen chloride to give acetic acid and acetyl chloride.
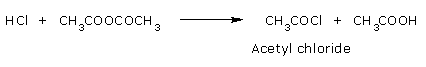
Reaction with Phosphorous penta chloride:
Acetic anhydride reacts with Phosphorous pentachloride to form Acetyl chloride.
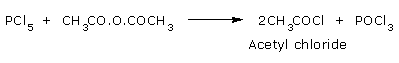
Halogenation:
Acetic anhydride reacts with chlorine to form mono chloro derivative.
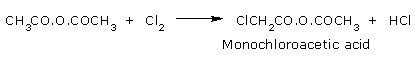
HCl formed further reacts to give chloro acetyl chloride and acetic acid.