How cane sugar is manufactured
Cane grows very tall in good growing regions – certainly up to 3 metres/10 feet tall – and still has some green leaves when ripe although most leaves have dried off by then. Where possible the cane is fired before harvesting to remove the dead leaf material and some of the waxy coating. The fire burns at quite high temperatures but is over very quickly so that the cane and its sugar content are not harmed.
In some areas
burning is not permitted because of the nuisance value to local communities of the smoke and
carbon specs that are released. However there is no environmental impact, the CO2
released being a very
small proportion of the CO2 fixed with photosynthesis during growth and the
improved sugar extraction
meaning that less cane needs to be grown on fewer acres to satisfy the world's sugar
demand.
Harvesting is done either by hand or by machine. Hand cut cane –– cane cutting is a hard and dirty job
but
can employ lots of people in areas where jobs are scarce –– is cut at about ground level, the top green
leaves are cropped off and then the stalk is bundled whole. Once a complete bundle has been assembled it
is
removed from the field with a light cart and may then be transferred to a larger vehicle for transport
to
the mill.
Most machine–cut cane is chopped into short lengths but is otherwise handled in a similar way as hand
cut
cane. Machines can only be used where land conditions are suitable and the topography is relatively
flat. In
addition the capital cost of machines and the loss of jobs caused makes this solution unsuitable for
many
sugar estates.
There are several important aspects to extraction which involve the energy balance of the factory, the
efficiency of extraction and therefore ultimately the profitability of operations:
The manager needs to process the cane as soon as possible if sugar losses are to be avoided yet needs
to
have a sufficient supply in storage for times when cutting and transport are stopped, whether
deliberately
or not. Typically, cane is processed within 24 hours of cutting
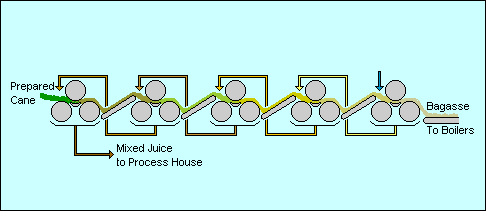
- Cane preparation is critical to good sugar extraction, particularly with diffusion extraction. This is achieved with rotating knives and sometimes hammer mills called "shredders". However shredding requires extra energy and more equipment
- The extraction is actually conducted as a counter–current process using fresh hot water at one end being pumped in the opposite direction to the cane. The more water that is used, the more sugar is extracted but the more dilute the mixed juice is and hence the more energy that is required to evaporate the juice
- The more accurately that the mills are set [adjusted], the drier is the residual fiber and hence the less sugar remaining in the fiber
A typical mixed juice from extraction will contain perhaps 15% sugar and
the residual fiber, called bagasse,
will contain 1 to 2% sugar, about 50% moisture and some of the sand and grit from the field
as "ash".
A typical cane might contain 12 to 14% fiber which, at 50% moisture content gives about 25
to 30 tons of bagasse per 100 tons
of cane or 10 tons of sugar.
The mixed juice from extraction is preheated prior to liming so that the clarification is optimal. The
milk
of lime, calcium hydroxide or Ca(OH)2, is metered into the juice to hold the required ratio and the
limed
juice enters a gravitational settling tank: a clarifier. The juice travels through the clarifier at a
very
low superficial velocity so that the solids settle out and clear juice exits.
The mud from the clarifier still contains valuable sugar so it is filtered on rotary vacuum filters
where
the residual juice is extracted and the mud can be washed before discharge, producing a sweet water. The
juice and the sweet water are returned to process.
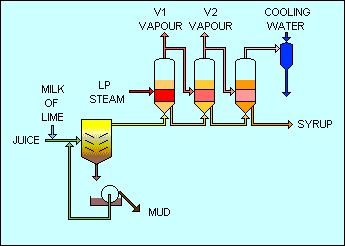
The clear juice has probably only 15% sugar content but saturated sugar liquor, required before
crystallization can occur, is close to 80% sugar content. Evaporation in a steam heated multiple effect
evaporator is the best way of approaching the saturated condition because low pressure water vapors can
be
produced for heating duties elsewhere in the factory.
The evaporator sets the steam consumption of the factory and is designed to match the energy balance of
the
entire site: the manager wants to avoid burning auxiliary fuel and equally wants to avoid paying to
dispose
of surplus bagasse. The greater the number of effects, the less steam is required to drive the first
effect.
Each subsequent effect is heated by the vapor from the previous effect so has to be operated at a lower
temperature and therefore lower pressure.
Boiling
Physical chemistry assists with sugar purification during the crystallization process because there is
a
natural tendency for the sugar crystals to form as pure sucrose, rejecting the non–sugars. Thus, when
the
sugar crystals are grown in the mother liquor they tend to be pure and the mother liquor becomes more
impure. Most remaining non–sugar in the product is contained in the coating of mother liquor left on the
crystals.
The mother liquor still contains valuable sugar of course so the crystallization is repeated several
times.
However non–sugars inhibit the crystallization. This is particularly true of other sugars such as
glucose
and fructose which are the breakdown products of sucrose. Each subsequent step therefore becomes more
difficult until one reaches a point where it is no longer viable to continue.
The crystallization step itself – a "boiling" – takes place in a vacuum pan: a large closed kettle with
steam heated pipes. [In practice the heating is done with a low pressure water vapor from the
evaporator.
Some modern pans are continuous flow devices but most are batch devices which go through a discrete
cycle
and are then emptied for a new boiling. A typical cycle might be 4 hours long. The mixture of crystals
and
mother liquor from a boiling, called the "massecuite", is dropped into a receiving tank called a
crystallizer where it is cooled down and the crystals continue to grow. This also releases the pan for a
new
boiling. From the crystallizer the massecuite is fed to the centrifuges.
In a raw sugar factory it is normal to conduct three boiling. The first or "A" boiling produces the
best
sugar which is sent to store. The "B" boiling takes longer and the retention time in the crystallizer is
also longer if a reasonable crystal size is to be achieved. Some factories re–melt the B sugar to
provide
part of the A boiling feedstock, others use the crystals as seed for the A boiling and others mix the B
sugar with the A sugar for sale. The "C" boiling takes proportionally longer than the B boiling and
considerably longer to crystallize. The sugar is usually used as seed for B boiling and the rest is
re–melted.
Storage
The final raw sugar forms a sticky brown mountain in the store and looks rather like the soft brown
sugar
found in domestic kitchens. It could be used like that but usually it gets dirty in storage and has a
distinctive taste which most people don't want. That is why it is refined when it gets to the country
where
it will be used. Additionally, because one cannot get all the sugar out of the juice, there is a sweet
by–product made: molasses. This is usually turned into a cattle food or is sent to a distillery where
alcohol is made.

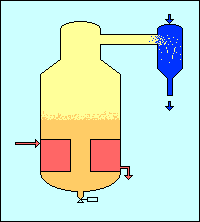
Extraction

Evaporation

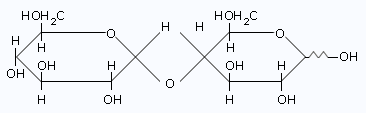
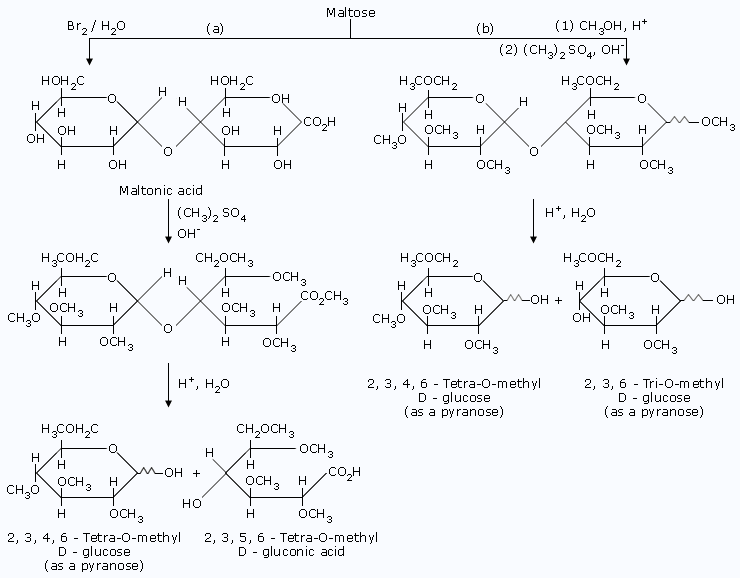
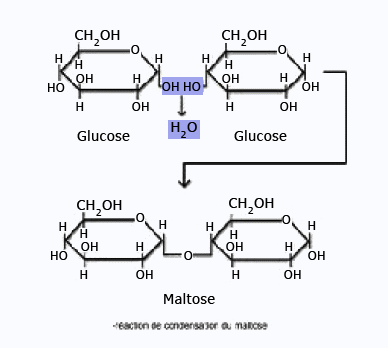
Maltose
The above facts demonstrate that one of the glucose residue of maltose is
present in a hemiacetal form;the other,
therefore,must be present as a glucoside.The configuration of this glucosidic linkage can be
inferred as α,
because maltose is hydrolyzed by α–glucosidase enzymes and not by
β–glucosidase enzymes.
- Maltose reacts with bromine water to form a monocarboxylic acid,maltonic acid.This fact,too,is consistent with the presence of only one hemiacetal group.
- Methylation of maltonic acid followed by hydrolysis gives,2, 3, 4, 6–tetra–O–methyl–D–glucose and 2, 3, 5, 6–tetra–O–methyl–D–gluconic acid.That the first product has a free –OH at C5 indicates that the non–reducing glucose portion is present as a pyranoside;that the second product,2, 3, 5, 6–tetra–O–methyl–D–gluconic acid,has a free –OH at C4 indicates that this position was involved in a glycosidic linkage with the non–reducing glucose. Only the size of the reducing glucose ring needs to be determined.
- Methylation of maltose itself,followed by hydrolysis gives 2, 3, 4, 6–tetra–O–methyl–D–glucose and 2, 3, 6–tri–O–methyl–D–glucose.The free –OH at C5 in the latter product indicates that it must have been involved in the oxide ring and that the reducing glucose is present as a pyranose.