Chain growth polymerization proceeds in three important steps :
- Initiation step:
This step involves the formation of a reactive particle. - Propagation step:
It consists of a growing polymer chain having reactive particles. - Termination step:
In this step, the growth of the chain is finally terminated.
In the free radical polymerization , monomer is activated by the action of light, heat, or by adding chemicals, known as initiators.
Examples of initiators are benzoyl per-oxide and azo
bisisobutyronitrile (AIBN).
The polymerization of styrene, initiated by benzoyl peroxide, is a typical example of free
radical polymerization.
- Initiation:
This consists of the decomposition of benzoyl peroxide into benzoyl oxy-free radicals
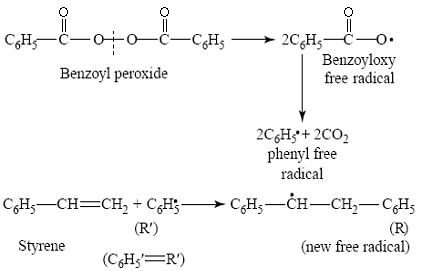
- Propagation:
In the extremely rapid chain-propagating step, the new free radical adds to the double bond of another styrene monomer and forms a new radical which is capable of further interacting with the initial styrene monomers and in this step macro–free radical is formed.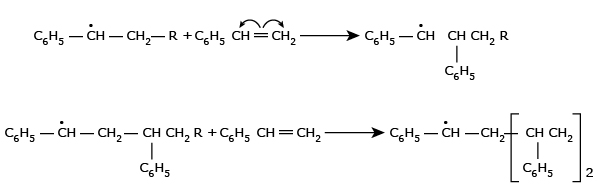
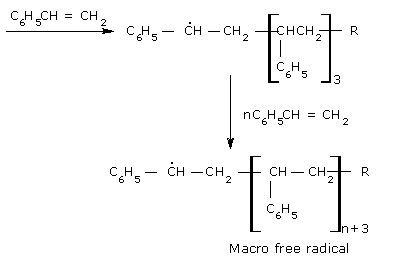
- Termination: The macro-free radicals are deactivated by one of the
following methods.
- Recombination of free radicals: The growing free radical
reacts with the other growing free radical.
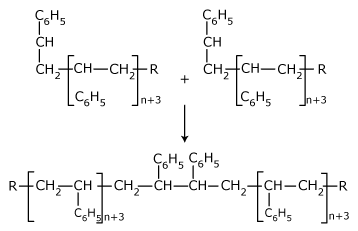
- Reaction with inhibitors: Polymeric chain can be
terminated by the reaction of inhibitors,
such as hydroquinone, phenol, amines, etc. For example, phenoxy
radicals(ArO°) derived from phenol (ArOH)
are highly resonance stabilized and so unreactive that they cannot initiate
chain reaction and thus,
polymeric chain is terminated

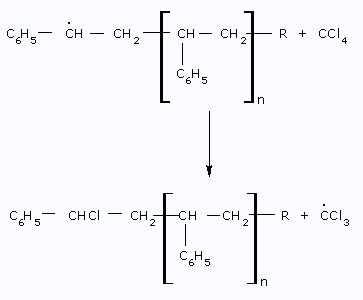
- Reaction with the solvent: The solvent molecule, such as
CCl4, reacts
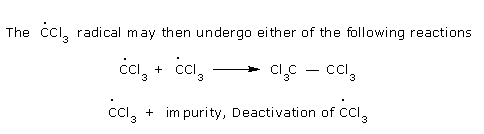
with the Radical and produces CCl3 radical.
- Recombination of free radicals: The growing free radical
reacts with the other growing free radical.
Cationic Polymerization (Carbocation Polymerization):
In cationic polymerization, the catalysts used are Lewis acids
(electron acceptor), such as SnCl4, TiCl4, AlCl3,
BF3 and H2SO4.
If the catalyst is not a strong protonic acid then the co-catalysts are required to provide
protons.
The polymerization proceeds through the formation of the carbocation and the
chain growth is accompanied with the transfer of the positive charge along with the polymer
chains. Its mechanism involves the following steps:
Step I: Chain Initiation If the acidic catalyst is not
a protonic acid then the co-catalyst is used to produce proton. 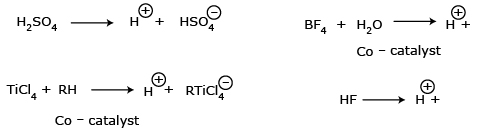 The produced proton adds to the multiple
bond of the monomer molecule and forms the carbocation.
The produced proton adds to the multiple
bond of the monomer molecule and forms the carbocation. 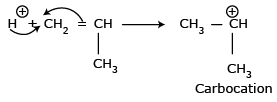
Step II: Chain Propagation The carbocation reacts with
another monomer molecule and chain growth takes place. 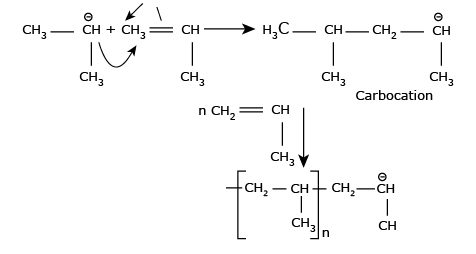
Step III: Chain Termination Chain termination can take
place by the combination with an anion or by loss of a proton. 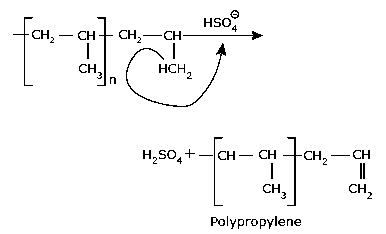
The essential feature of these polymerizations is that they take place at
very high rates even at low temperature.
Example: Polymerization of isobutene is completed in a few seconds at - 100°C.
Anionic Polymerization (Carbanion Polymerization):
Anionic polymerization involves the formation of carbanion.
In these polymerizations, the electron-donor catalysts are used.
Example: alkali metal alkyls, alkali metal amides and Grignard reagents.
Step I: Initiation Step 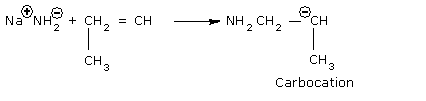
Step II: Propagation Step 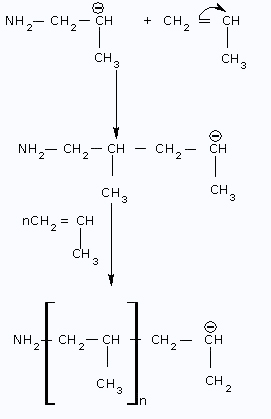
Step III: Termination Step 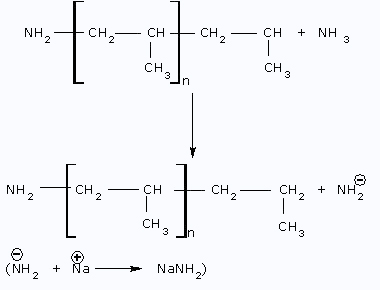 Anionic polymerization is more favorable
with the monomers having electron withdrawing substituents
(e.g. CN-), since such groups help in the stabilization of the
intermediate anion.
Anionic polymerization is more favorable
with the monomers having electron withdrawing substituents
(e.g. CN-), since such groups help in the stabilization of the
intermediate anion.
In case of anionic polymerization, if some other molecule or an impurity is not added, termination step cannot take place. Reaction stops, when all monomer is consumed.
Hence, polymers produced by this method retain active centers at the end of the chain. Such polymers are known as living polymers. These living polymers may further react with the additional monomer molecule and may be killed by the addition of a terminating agent. So this type of polymerization may lead to polymers of desired low molecular weight.
Examples: Derivatives of acrylonitrile, acrylic and methacrylic esters, ethylene, styrene and butadiene are the common examples of the monomers polymerized by an-ionic polymerization reaction.