Examples of catalysts
The finely–divided nickel can adsorb fairly large quantity of hydrogen especially at high temperatures.
Due to this property,finely–divided Ni is used as a catalyst
in various reactions.
Reduction of unsaturated ethylene compounds(Sabatier process)
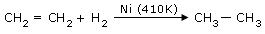
In the hydrogenation of oils.
In this process the unsaturated oils are converted into
saturated fats(hardening of oils)in presence of Ni.
The following hydrogenation reactions are catalyzed by heated nickel.
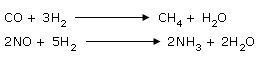
Platinum black is used as a catalyst in the preparation of HCHO from CH3OH and in
the decomposition of H2O2.
Copper is also used as a catalyst for
converting CH3OH to HCHO
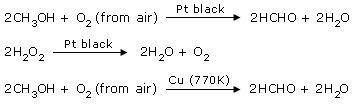
Fenton's reagent(FeSO4 + H2SO4) is used for oxidizing
alcohols to aldehydes.
Co(II) salts catalyze the decomposition of bleaching powder (ClO−) as
Co(II) salts can easily be oxidized to Co(III)salts.
