 Polymers - Combination of similar monomers
The word "Polymer" is generated from the Greek words "Polys + Meris" (English: Many + Parts).
Polymers - Combination of similar monomers
The word "Polymer" is generated from the Greek words "Polys + Meris" (English: Many + Parts).
The multitude fields of biochemistry, medicine, and molecular biology have been profoundly influenced by discoveries in polymer chemistry. In exploring the relationship between the three-dimensional structures of biomolecules and their incredible biological function, biochemists have elucidated how nerve impulses travel with incredible speed, how enzymes accurately catalyze biological reactions, and the molecular mechanisms underlying many diseases. An understanding of polymers helped elucidate how DNA and RNA molecules store and transmit genetic information and direct the synthesis of proteins- the body building blocks! The understanding of the structure and molecular function of proteins stands as one of the greatest achievements of modern science and is still a highly active area of research. In this topic, we shall briefly consider the structure and function of polymers.
Simple organic molecules are likely to be produced from elements such as carbon, oxygen, hydrogen and nitrogen present in earth’s atmosphere under the influence of ultraviolet radiations of sun and lighting discharges. When these small organic molecules are joined together, "giant" molecules are produced. These giant molecules are known as macromolecules.
Macromolecules are polymers (from the Greek polys - many, and meris - part). For example: The large molecules of carbohydrates (starch), proteins (Hemoglobin) and nucleic acids (DNA and RNA) are polymers. For example, Polymers are large molecules of many similar "units" linked together by covalent bonds. The repeating units that serve as the building blocks of a polymer are small molecules called monomers.
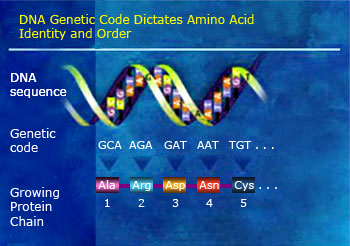
DNA polymer is formed from nucleic acid monomers. Human DNA is a polymer with over 20 billion constituent atoms. Protein polymer is formed from amino acid monomers. Proteins are built from 20 kinds of amino acids arranged in chains that are typically hundreds of amino acids long.