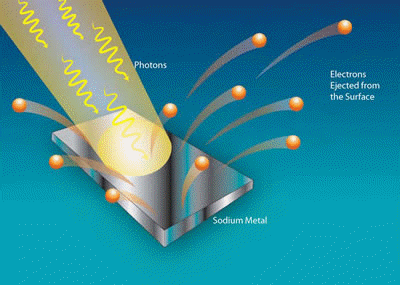
 Photo–electrons Emitting from a Sodium Metal due to
Photo Electric Effect.
Photo–electrons Emitting from a Sodium Metal due to
Photo Electric Effect.
When a surface is exposed to sufficiently energetic electromagnetic energy, light will be absorbed and electrons will be emitted.
This process is called the photoelectric effect (or photoelectric emission or photoemission), a material that can exhibit this phenomena is said to be photoemissive, and the ejected electrons are called photo–electrons. The photoelectric effect occurs with photons having energies from a few electron volts to over 1 MeV.
Laws of Photoelectric Emission:
- Photoelectric current is directly proportional to the intensity of light, provided the frequency of radiation is above the threshold frequency.
- For a given material, there is a certain minimum (energy) frequency, called threshold frequency, below which the emission of photo electrons stops completely, no matter how high is the intensity of incident light.
- The maximum kinetic energy of the photo–electrons is found to increase with increase in the frequency of incident light, provided the frequency exceeds the threshold limit.
- The photo–emission is an instantaneous process. It just takes around 10 – 9s for the ejection of photo–electrons as the photons strikes the metal surface.