Worked Out Example
A neutral water molecule (H2O) in its vapour state has an electric dipole moment of magnitude 6.2 × 10–30 C.m. How far apart are the molecule's centers of positive and negative charge? If the molecule is placed in an electric field of 1.5 × 104 N/C, what maximum torque can the field exert on it ?
sol: A molecule's dipole moment depends on the magnitude q of the molecule's positive or negative charge and the charge separation d.
There are 10 electrons and 10 protons in a neutral water molecule, so the magnitude of its dipole moment is p = qd =(10e)(d) in which d is the separation we are seeking and e is the elementary charge.
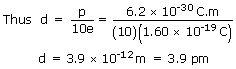
The key idea here is that the torque on a dipole is maximum when the
angle θ between 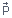 and
and  is 90°.
is 90°.
τ = pE sinθ
τ = (6.2 × 10– 30 C.m)(1.5 × 104 N/C) (sin 90°)
τ = 9.3 × 10– 26 N.m