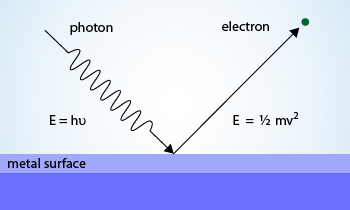
 Photoelectric effect on metal surface
Photoelectric effect on metal surface
Photoelectric effect :
The mathematical theory of electromagnetism by James Clerk Maxwell
led to the view that light is of electromagnetic nature, propagating as a wave from the source to the receiver. The discovery of
X–rays by Wilhelm Conrad Rontgen in 1895 had shown among many other things that X–rays like light,
were propagating in straight lines but in contrast to light penetrating through matter. Reflection, refraction, diffuse scattering,
polarization, diffraction, emission and absorption spectra, photoelectric effect, all of the essential characteristics of light
have been found also to be characteristics of X–rays. Albert Einstein explained the Photoelectric effect, i.e., the emission
of electrons from metal surfaces illuminated by light with particle theory as wave theory of light was unable to explain the
phenomena.
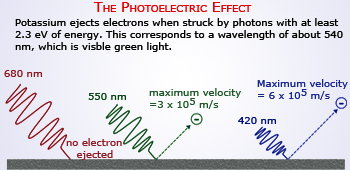 The Photoelectric effect
The Photoelectric effect
Einstein proposed the law that an electron emitted from a substance by monochromatic light with the frequency has to have a maximum energy of E = h − p, where p is the energy needed to remove the electron from the substance. Only photons of a high – enough frequency, (above a certain threshold value) could knock an electron free. For example, photons of blue light had sufficient energy to free an electron from the metal, but photons of red light did not. More intense light above the threshold frequency could release more electrons, but no amount of light below the threshold frequency could release an electron. Robert Andrew's Millikan carried out a series of measurements over a period of 10 years, finally confirming the validity of this law in 1916 with great accuracy.
The most important application of Einstein's photoelectric law and also its most convincing confirmation has come from the use Bohr made of it in his theory of atoms, which explains a vast amount of spectroscopic data.
Photon emission (or light emission) from atoms involves the transitions of electrons from higher to lower energy states within the atom. Each element possesses its own characteristic pattern of electron shells or energy states. Because these states can only have certain energies, we say they are discrete. We call these discrete states as quantum states.
 Early photoelectric cell
Photoelectric cells are used to convert light falling on them into electricity. This type of photoelectric
cell was developed specifically for early television transmitters. Photoelectric cells make use of an effect whereby metals emit
electrons when exposed to light (photo–emissivity). A metal plate of either rubidium, potassium or sodium (the cathode) is
enclosed in a vacuum and connected to the negative pole of a battery. A loop of wire (the anode) faces the plate. When exposed to
light, electricity crosses the gap between the cathode and anode more easily, enabling the cell to produce impulses of electricity
dependent on the intensity of the light falling on it.
Early photoelectric cell
Photoelectric cells are used to convert light falling on them into electricity. This type of photoelectric
cell was developed specifically for early television transmitters. Photoelectric cells make use of an effect whereby metals emit
electrons when exposed to light (photo–emissivity). A metal plate of either rubidium, potassium or sodium (the cathode) is
enclosed in a vacuum and connected to the negative pole of a battery. A loop of wire (the anode) faces the plate. When exposed to
light, electricity crosses the gap between the cathode and anode more easily, enabling the cell to produce impulses of electricity
dependent on the intensity of the light falling on it.
If a photon of light is absorbed by the electron, the electron gains potential energy and moves farther from nucleus. It means that the electron is raised to a higher energy level and the atom is said to be excited. The electron's higher position is only momentary. The electron loses its temporarily acquired energy and a photon of light is emitted by the electron and the electron loses potential energy and moves closer to nucleus. It means that the electron returns to a lower level and emits radiant energy. Now the atom is said to be de–excited.
The frequency of the emitted photon is related to the energy transition of the jump and is directly proportional
to its energy. When the proportionality constant his introduced, this becomes the exact equation, (E1 −
E2) = h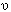 where h is
Planck's constant.
where h is
Planck's constant.
Bohr used the law of Einstein as the basic condition for the frequency of light emitted or absorbed, when an atom makes a transition between two quantized energy levels E1 and E2, in the form
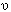 = (E1 −
E2)/h, which in modern texts is nothing but basic energy conservation with a photon emitted or absorbed as the case may
be.
= (E1 −
E2)/h, which in modern texts is nothing but basic energy conservation with a photon emitted or absorbed as the case may
be.