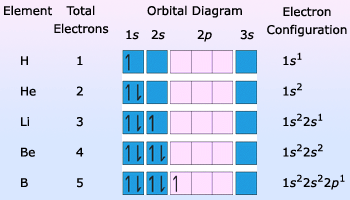
 Table Showing Filling Of Electrons In Orbitals
Table Showing Filling Of Electrons In Orbitals
Electronic configurations:The arrangement of electrons in the orbitals of an atom is called the atom's electronic configuration. The electronic configuration of any atom can be shown in an energy–level diagram by placing that atom's electrons in the orbitals in the order of increasing energy level. The pairing of electrons of opposite spin in an orbital takes place only when necessary.
An abbreviated way of presenting electronic configuration is to write the principal quantum number and letter of each occupied orbital and then use a superscript to indicate number of electrons in each orbital. The orbitals of each atom are then written in the order of increasing energy levels.
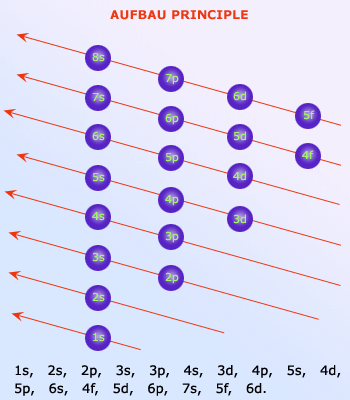 Arrangement of electrons is done according to the principle proposed by Aufbau. Electrons must be filled
according to the arrow marks as shown in the figure. The Aufbau principle works for nearly every element discovered. There are
few exceptions to this principle, chromium and copper deviate from it.
Arrangement of electrons is done according to the principle proposed by Aufbau. Electrons must be filled
according to the arrow marks as shown in the figure. The Aufbau principle works for nearly every element discovered. There are
few exceptions to this principle, chromium and copper deviate from it.
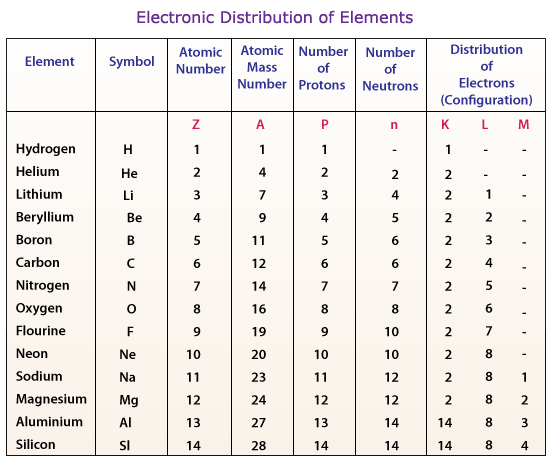
Distribution of electrons in principal shells of some elements is given below:
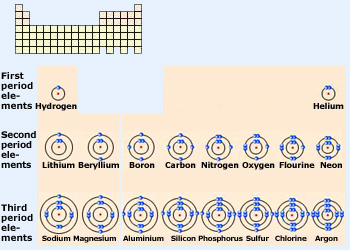 The first three periods of the periodic table according to the shell model. Elements in the same period
have electrons in the same shells. Elements in the same period differ from one another by the number of electrons in the outermost
shell.
The first three periods of the periodic table according to the shell model. Elements in the same period
have electrons in the same shells. Elements in the same period differ from one another by the number of electrons in the outermost
shell.
The electrons filling different atomic energy levels or shells, is neatly given by Aufbau principle, shown in the adjacent figure.
All the superscripts for an atom must add up to the total number of electrons in the atom – 1 for hydrogen, 3 for lithium, 11 for sodium, and so on. The orbitals are not always listed in order of principal quantum number. The 4s orbital, for example, is lower in energy than the 3d orbitals as is indicated on the energy level diagram. The 4s orbital, therefore, appears before the 3d orbital.
Mostly the outermost electrons, the ones farthest from the nucleus, determine the properties of an atom. These are the electrons towards the “outer surface” of the atom and hence the ones in direct contact with the external environment. Elements that have similar electronic configurations in their outermost orbitals, therefore, have similar properties. In a shell diagram, the electrons are filled in just as in an energy–level diagram, electrons first fill the shells closest to the nucleus. Electrons do not begin to pair in a shell until the shell is half filled. Adjacent figure shows how this works for the first three periods. As with energy–level diagrams, there is one shell for each period, and the number of elements in a period is equal to the maximum number of electrons the shells representing that period can hold.