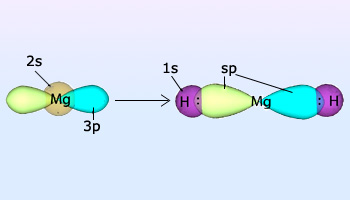
 Formation of MgH2 as a result of overlap between sp and s orbital of ‘H’ atom.
Formation of MgH2 as a result of overlap between sp and s orbital of ‘H’ atom.
Intermixing of one ‘s’ and one ‘p’ orbitals of almost equal energy to give two identical and degenerate hybrid orbitals is called ‘sp’ hybridization.
These sp–hybrid orbitals are arranged linearly at by making 180° of angle. They possess 50% ‘s’ and 50% ‘p’ character.
Example: Magnesium hydride
In magnesium hydride, the 3s orbital and one of the 3p orbitals from magnesium hybridize to form two sp orbitals. The two frontal lobes of the sp orbitals face away from each other forming a straight line leading to a linear structure. These two sp orbitals bond with the two 1s orbitals of the two hydrogen atoms through sp–s orbital overlap.