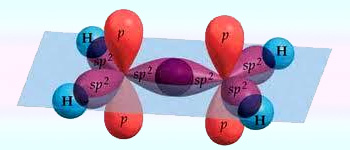
 sp2 hybrization showing overlapping of hybridised orbitals
sp2 hybrization showing overlapping of hybridised orbitals
Intermixing of one ‘s’ and two ‘p’ orbitals of almost equal energy to give three identical and degenerate hybrid orbitals is known as sp2 hybridization.
The three sp2 hybrid orbitals are oriented in trigonal planar symmetry at angles of 120° to each other. The sp2 hybrid orbitals have 33.3% ‘s’ character and 66.6% ‘p’ character.
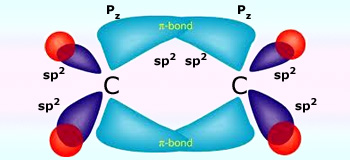 sp2 hybridization in Ethene
sp2 hybridization in Ethene
Similar hybridization occurs in each carbon of ethene. For each carbon, one 2s orbital and two 2p orbitals hybridize to form three sp2 orbitals. These hybridized orbitals align themselves in the trigonal planar structure. For each carbon, two of these sp orbitals bond with two 1s hydrogen orbitals through s–sp2 orbital overlap. The remaining sp2 orbitals on each carbon are bonded with each other, forming a bond between each carbon through sp2 – sp2 orbital overlap. This leaves us with the two p orbitals on each carbon that has a single carbon in them. These orbitals form a π bonds through p–p orbital overlap, creating a double bond between the two carbons. Because a double bond was created, the overall structure of the ethene compound is linear. However, the structure of each molecule in ethene, the two carbons, is still trigonal planar.