 Copper sulfate solution
Mass percentage of copper sulfate in Copper sulphate solution is:
Copper sulfate solution
Mass percentage of copper sulfate in Copper sulphate solution is: 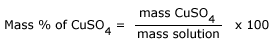
Several concentration terms are based on the number of solute (or solvent) parts present in a specific number of solution parts. The solution parts can be expressed in terms of mass, volume, or amount (mol).
Parts by mass: The most common of the parts by mass terms is mass percent. The word percent means “per hundred” so mass percent of solute means the mass of solute dissolved in every 100 parts by mass of solution, or the mass fraction times 100:
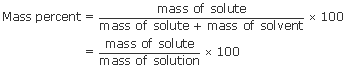
Sometimes, mass percent is symbolized as % (w/w), indicating that the percentage is the ratio of weights (more accurately, masses). Two very similar terms are parts per million (ppm) by mass and parts per billion (ppb) by mass: grams of solute per million or per billion grams of solution. For these quantities, in above equation, you multiply by 106 or by 109, respectively, instead of by 100.