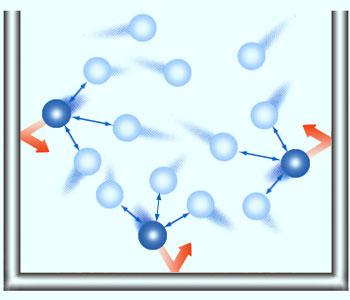
 Pressure of real gases will be lower than expected because of intermolecular forces of attraction operating in between the molecules. Particles take curved paths on collisions and takes time to reach the walls. This reduces the collision frequency, lowering the pressure of the gas.
Pressure of real gases will be lower than expected because of intermolecular forces of attraction operating in between the molecules. Particles take curved paths on collisions and takes time to reach the walls. This reduces the collision frequency, lowering the pressure of the gas.
The redesigning of ideal gas law: ohannes van der Waals developed a modification of the ideal gas law to deal with the non–ideal behaviour of real gases.
He reasoned that, if the gas particles each occupied some volume, there would be a net decrease in the useful volume of the container. This decrease in volume must be proportional to the moles of gas particles present. By using the letter ‘b’ for the proportionality constant, the volume term in the ideal gas law could be replaced by (V–nb). In evaluating the effect of intermolecular attractions, it was reasoned that, if gas particles attracted each other, they would have curved paths and therefore would take longer time to collide with the container walls. The result would be a decreased collision frequency and a lower pressure for the real gas than the pressure of an ideal gas under the same conditions. The frequency of attractions between gas particles would be expected to rise with greater concentrations of the gas. The best correction factor was found to be based on the square of the concentration of the gas, (n/V)2. The proportionality constant was given the symbol ‘a’, and the entire correction factor was added to the measured pressure [P + a (n/V)2] to obtain the equivalent ideal pressure.
| Gas | 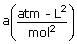 |
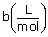 |
|---|---|---|
| He | 0.03421 | 0.02370 |
| Ne | 0.2107 | 0.01709 |
| Ar | 1.345 | 0.03219 |
| Kr | 2.318 | 0.03978 |
| Xe | 4.194 | 0.05105 |
| H2 | 0.0244 | 0.02661 |
| O2 | 1.360 | 0.03183 |
| N2 | 1.390 | 0.03913 |
| CH4 | 2.253 | 0.04278 |
The van der Waals equation for real gases takes the form
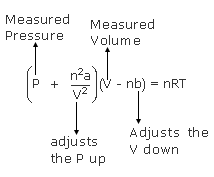
The value of the proportionality constant ‘a’ represents the relative strength of attractive forces acting between the gas molecules, with larger values of ‘a’ indicating stronger attractive forces such as dipole–dipole attractions. The value of the constant ‘b’ represents the relative size of the gas molecule. The larger the value of ‘b’, the larger is the size of the molecule. The size of gas molecules does not vary greatly from one molecule to another, and this contribution to deviations from the ideal gas law is similar for many molecules. Attractive forces, however, vary greatly, depending on molecular polarity, and contribute the most to deviations from ideal gas law.