 Any gas irrespective of its nature
occupies 22.4L at standard temperature and pressure conditions.
Any gas at 0°C and 1 atm pressure occupies the same
volume irrespective of its chemical composition. In 1982,
IUPAC has standardized the values as 0°C and 100 kPa
(1 bar) as conditions for standard temperature and pressure.
Any gas irrespective of its nature
occupies 22.4L at standard temperature and pressure conditions.
Any gas at 0°C and 1 atm pressure occupies the same
volume irrespective of its chemical composition. In 1982,
IUPAC has standardized the values as 0°C and 100 kPa
(1 bar) as conditions for standard temperature and pressure.
A gas possesses neither shape nor volume of its own. It occupies the whole volume of the container. When a gas is heated, both its volume and pressure can change depending upon the conditions. For this reason, the behavior of a gas is always considered in two ways:
- The pressure is kept constant and the effect of temperature on the volume of the gas is considered.
- The volume is kept constant and the effect of temperature on the pressure of the gas is considered.
Some relations known as "Gas laws" express the behavior of gases. These are Charles's law, Gay–Lussac's law and Boyle's law.
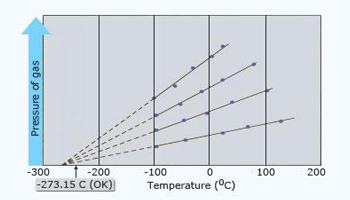 Plot of pressure v/s temperature
Each line shows how the pressure varies with temperature.
Plot of pressure v/s temperature
Each line shows how the pressure varies with temperature.
Gay–Lussac's law:
At constant volume, the pressure of a gas is directly proportional to its absolute temperature. If P is the pressure of a gas
and T is its absolute temperature, then at constant volume, P ∝ T.
The application of this law can be experienced in our daily life. Someone opening an oven may feel a quick flow of hot air; the air inside the oven is heated, therefore pressurized.
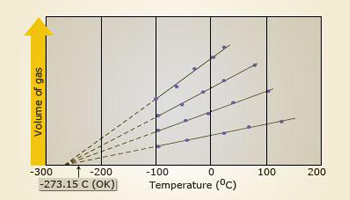 Plot of volume v/s temperature
Each line shows how the
gas volume varies with temperature for different volumes of same gas.
Plot of volume v/s temperature
Each line shows how the
gas volume varies with temperature for different volumes of same gas.
Charles's law:
At constant pressure, the volume of a gas is directly proportional
to its absolute temperature. If V is the volume of a gas and T is its absolute temperature, then at constant pressure, V∝ T.
An example is weather balloons are launched daily from weather stations across the country. The balloon begins at the earth at certain pressure, temperature, and volume and upon its accent all three of these variables change in response to the surroundings.
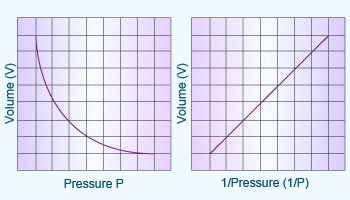 Boyle's law PV= constant
The variation of volume with pressure
at constant temperature for a fixed amount of gas.
Boyle's law PV= constant
The variation of volume with pressure
at constant temperature for a fixed amount of gas.
Boyle's law:
At a constant temperature, the volume of a given mass of gas is
inversely proportional to its pressure.
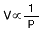 or
or
P V = constant
In other words, P1V1 = P2 V2 where P1, V1 and P2, V2 are values of pressure and volume at temperature T1 and T2 respectively.
Many gases are stored in high pressure. In this way they would occupy a small volume. This is an application of Boyle's law. For example, some cars use compressed natural gas as fuel.
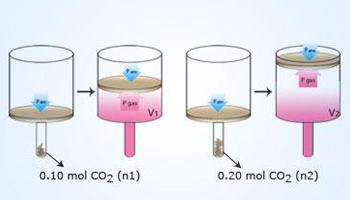 An experiment to study the relationship
between the volume and amount of a gas. At a given external
P and T, a given amount (n1) of CO2(s) is put
into the tube. When the CO2 changes from
solid to gas,it pushes up the piston until Pgas = Patm,
point at which it occupies a given volume of the cylinder.
When twice the amount (n2) of CO2 (s)
is used, twice the volume of the cylinder becomes occupied.
Thus, at fixed P and T, and volume (V) of a gas is directly
proportional to the amount of gas (n).
An experiment to study the relationship
between the volume and amount of a gas. At a given external
P and T, a given amount (n1) of CO2(s) is put
into the tube. When the CO2 changes from
solid to gas,it pushes up the piston until Pgas = Patm,
point at which it occupies a given volume of the cylinder.
When twice the amount (n2) of CO2 (s)
is used, twice the volume of the cylinder becomes occupied.
Thus, at fixed P and T, and volume (V) of a gas is directly
proportional to the amount of gas (n).
Avogadro's Law:
The volume of a gas maintained at constant temperature and
pressure is directly proportional to the number of moles of gas. That is, V = constant × n. Thus, doubling the number of
moles of gas will cause the volume to double if T and P remain constant.
Dalton's law of partial pressures states:
The total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases.
Pressure Total = Pressure Gas1 + Pressure Gas2 + Pressure Gas3 + ... Pressure Gasn
If the total pressure is known and the number of moles of each component gas are known, the partial pressure can be computed
using the formula:
Px = Ptotal (nx / nTotal)
where
Px = partial pressure of gas × Ptotal = total pressure of all gases nx = number of moles
of gas × nTotal = number of moles of all gases. This relationship applies to ideal gases, but can be used in
real gases with very little error.
Graham's Law:
Graham's Law is a relation which states that the rate of the
effusion of a gas is inversely proportional to the square root of its density or molecular mass.
Rate1 / Rate2 = (M2/M1)1/2
where:
Rate1 is the rate of effusion of one gas, expressed as volume or as moles per unit time.
Rate2 is the rate of effusion of the second gas.
M1 is the molar mass of gas 1.
M2 is the molar mass of gas 2.