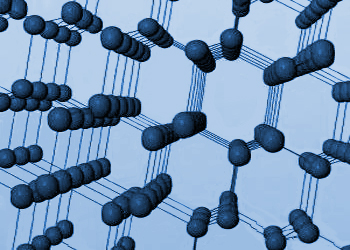
 Two types of forces exist in nature.
Intramolecular forces are strong when compared with intermolecular forces
due to influence of several forces operating between them. An extended
3–Dimensional lattice of diamond which is very strong due to
covalent bonding between the carbon atoms.
Two types of forces exist in nature.
Intramolecular forces are strong when compared with intermolecular forces
due to influence of several forces operating between them. An extended
3–Dimensional lattice of diamond which is very strong due to
covalent bonding between the carbon atoms.
 Examples of network solids
Examples of network solids
Forces of attraction: Molecular forces are forces of attraction or repulsion which act between neighboring particles i.e., atoms, molecules or ions. These forces are weak when compared to the intra–atomic forces that exist in the nucleus which are responsible for keeping the molecule together.
These forces can be classified into 2 types:
1) Intramolecular forces.
2) Intermolecular forces.
Intramolecular forces:
These are relatively strong forces when compared to the other forces
existing between the molecules. These are of 3 types. They are:
1) Covalent forces: These are considered to be the strongest forces among the molecular attractions. Covalent forces are responsible to hold the atoms together in lattice structure.
- These forces are the result of sharing of pairs of electrons between the atoms.
- Network solids(example: diamond, SiO2) are very hard and their strong bonds result in very high melting and boiling points.
- Network solids have localized electrons which are in fixed positions in the covalent bonds. This makes network solids poor conductors of electricity.
e.g: Diamond, Quartz – SiO2, Silicon carbide – SiC
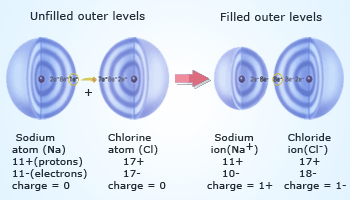 Ionic bond formation due to transfer of electrons.
Ionic bond is formed between Na+
and Cl– due to the transfer of electron from sodium
to chlorine.
Ionic bond formation due to transfer of electrons.
Ionic bond is formed between Na+
and Cl– due to the transfer of electron from sodium
to chlorine.
2) Ionic forces: These are the moderate
strong forces of attraction. In this type of forces adjacent ions have
electrostatic attractions resulting in an arranged lattice structure.
Ionic bonds operate between a cation, usually a metal and an anion
usually a non metal. Pure ionic forces cannot exist, all ionic compounds
will have some amount of covalent forces. Thus, an ionic force is considered
as a force where the ionic character is more than the covalent character.
Larger the electronegativity difference between the participating ions
stronger is the bond formed between the ions.
Coulomb's law states that the forces of attraction between two
objects is equal to the product of their charges
(q1 and q2) divided by the square of the
distance (r) between them.
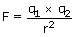
Charge on the ions is directly proportional
to the strength of ionic force. Size of the ions in the bond
is inversely proportional to the strength of ionic force.
e.g: NaCl, LiCl, CsCl, CsF, KI etc.,.
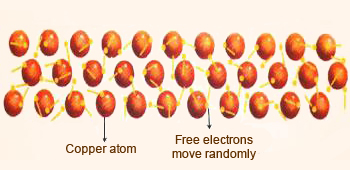 Metallic bonding in copper.
Metallic bonding in copper results
in presence of a delocalized electron which makes Cu a good
conductor of heat and electricity.
Metallic bonding in copper.
Metallic bonding in copper results
in presence of a delocalized electron which makes Cu a good
conductor of heat and electricity.
3) Metallic forces:
A metal is a lattice of positive metal ions in a sea of delocalized
electrons. Metallic bonding/force refers to the interaction between the
delocalized electrons and the metal nuclei. The physical properties
of metals are the result of the delocalization of electrons involved
in the metallic bonding. Valence electrons in metals are rampant,
they are not restricted to certain atoms or bonds. Rather they
run freely in the entire solid, providing good conductivity for
heat and electricity. These forces result in special properties
such as ductility and mechanical strength.
E.g: All metal elements Cu, Fe, Zn etc.,
Three types of intermolecular attractive forces are known to exist between neutral molecules and molecules involving dipole–dipole forces, London dispersion forces, and hydrogen bonding forces. Another kind of attractive force, the ion–dipole force, is important in solutions. As a group, intermolecular forces tend to be less than 15 percent as strong as covalent or ionic bonds. As we consider these forces, notice that each is electrostatic in nature, involving attractions between positive and negative species.