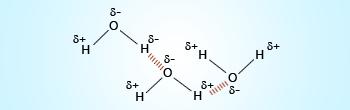
 Hydrogen bonding in water.
Water exists as liquid as a result of hydrogen bonding. Without hydrogen bonding water would have been
present in nature as gas at room temperature. Thus, hydrogen bonding is the gift of nature to mankind.
Hydrogen bonding in water.
Water exists as liquid as a result of hydrogen bonding. Without hydrogen bonding water would have been
present in nature as gas at room temperature. Thus, hydrogen bonding is the gift of nature to mankind.
A hydrogen bond is a type of attractive (dipole–dipole) interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom. This bond always involves a hydrogen atom. Hydrogen bonds can occur between molecules or within parts of a single molecule. A hydrogen bond tends to be stronger than van der Waals forces, but weaker than covalent bonds or ionic bonds.
Hydrogen bonding in water is a relatively strong association (∼23kJ/mol) and is responsible for many of water's unique characteristics; i.e. high boiling point, high surface tension, expansion of the solid state, and ability to dissolve ionic and polar molecules.
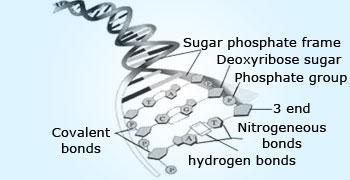 Hydrogen bonding in DNA.
Hydrogen bonding is one of the reasons for the double helical structure of DNA. The carboxylic group of one
amino acid and amine group present in its complimentary amino acid will result in hydrogen bonding holding the two strands of
the DNA together. Thus, hydrogen bonding plays a crucial role in structural aspects of molecules that are responsible for life.
Hydrogen bonding in DNA.
Hydrogen bonding is one of the reasons for the double helical structure of DNA. The carboxylic group of one
amino acid and amine group present in its complimentary amino acid will result in hydrogen bonding holding the two strands of
the DNA together. Thus, hydrogen bonding plays a crucial role in structural aspects of molecules that are responsible for life.
The density of ice at 0°C (0.917 g/mL) is less than that of liquid water at 0°C (1.00 g/mL). The low density of ice compared to that of water can be understood in terms of hydrogen–bonding interactions between water molecules. The interactions in the liquid are random. However, when water freezes, the molecules assume the ordered, open arrangement, which leads to a less dense structure for ice compared to that of water. A given mass of ice occupies a greater volume than does the same mass of liquid water. The structure of ice permits the maximum number of hydrogen–bonding interactions between the H2O molecules.
The density of ice compared to water profoundly affects life on Earth. Because ice is less denser than water, ice floats. When ice forms in cold weather, it covers the top of the water, thereby insulating the water below, if ice were more denser than water, ice forming at the top of a lake would sink to the bottom, and the lake could freeze solid. Most aquatic life could not survive under these conditions. The expansion of water upon freezing is also what causes water pipes to break in freezing weather.