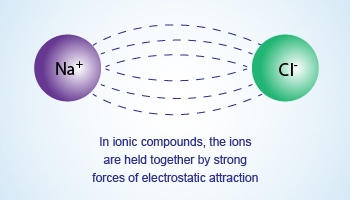
 Electrostatic forces of attraction
Electrostatic forces of attraction are responsible for holding the ions with strong forces making a
stable molecule. Lines of attractive forces can be seen in between the combining atoms.
Electrostatic forces of attraction
Electrostatic forces of attraction are responsible for holding the ions with strong forces making a
stable molecule. Lines of attractive forces can be seen in between the combining atoms.
Molecules and Motion: The credit for indirectly observing the existence of molecules first goes to Robert Brown, a Scottish botanist.
In 1827, Brown observed under a microscope
that pollen grains, which were of the size 10.5 m, when suspended
in water appeared to perform random motions. This type of random
motion of particles is termed as Brownian motion. Zigzag motion
of small particles can be seen in our everyday lives Einstein developed
the theory of Brownian motion in 1905. He explained that random motion
of pollen grains in water was due to the motion of water particles themselves.
Further, though it was known that the matter is made up of molecules,
the discovery of Brownian motion and Einstein's explanation of the same
gave the first direct evidence of the presence of small physical particles,
molecules, undergoing random motion.
Atoms combine to form molecules. Three atoms of hydrogen (H) combine
with a single atom of nitrogen (N) to produce ammonia molecule (NH3).
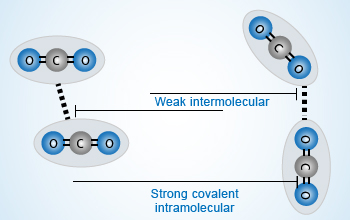 Forces of attraction
Two types of forces exist in nature. Intramolecular forces are strong when compared with intermolecular
forces due to influence of several forces operating between them.
Forces of attraction
Two types of forces exist in nature. Intramolecular forces are strong when compared with intermolecular
forces due to influence of several forces operating between them.
Forces of attraction:
When two atoms are brought together and as the distance between them
becomes comparable with their dimension, the positive and negative
charges of each atom start influencing charges in the other atom. This
gives rise to an interatomic force and changes the total energy of the pair.
Similar mechanism also gives rise to intermolecular interaction.
Knowing the size and the number of atoms and molecules, the next natural
curiosity is how they interact with one another. Clearly, the interaction
between molecules decides the properties of matter.
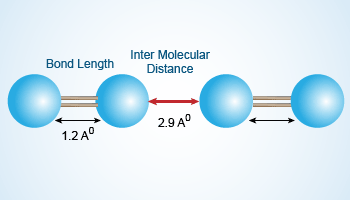
In an NaCl molecule, the Na atom donates an electron to the Cl atom to form an ionic bond. When large distances separate Na and Cl atoms, there is no electron transfer. When the Na and Cl atoms are at a distance of about 2.8 Å, the electron transfer takes place. This distance is the bond length of the NaCl atom.
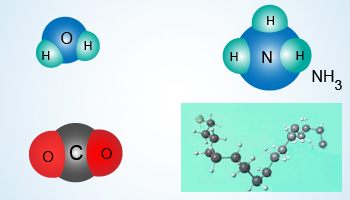 Different shapes of molecules due to differences in bond angles
Different shapes of molecules due to differences in bond angles
Besides having finite sizes, the molecules also have definite shapes.
- A water molecule has bond angles in such a manner that the molecule has an oxygen atom in the center with two hydrogen bonds at an angle of about 104.28°.
- An ammonia (NH3) molecule is tetrahedral in shape.
- A CO or a CO2 molecule is linear in shape.
- Hydrocarbons such as a sucrose molecule or a polyethylene molecule are long chain molecules.
The shape is determined by the minimum energy configuration of atoms that make up the molecule. Large molecules, such as those of hydrocarbons, also show that the bond lengths and the directions of the bonds govern their shape. The minimum potential energy thus depends on the bond length, the orientation of the bonds and the size of the molecules.
 Inter–molecular forces
Inter–molecular forces between the molecules would be very weak when compared with forces within
the molecule. It is easy to break the bond between the two oxygen molecules than between the two oxygen atoms as the forces of
attraction is directly proportional to 1/r6.
Inter–molecular forces
Inter–molecular forces between the molecules would be very weak when compared with forces within
the molecule. It is easy to break the bond between the two oxygen molecules than between the two oxygen atoms as the forces of
attraction is directly proportional to 1/r6.
Molecular forces are forces of attraction or repulsion which act between neighboring particles i.e., atoms, molecules or ions. These forces are weak when compared to the intra–atomic forces that exist in the nucleus which are responsible for keeping the molecule together.
These forces can be classified into 2 types:
- Intramolecular forces: Intramolecular force of attraction are the forces of attraction which keep a molecule together.
- Intermolecular forces: Intermolecular forces are forces of attraction or repulsion which act between neighboring particles (atoms, molecules or ions).