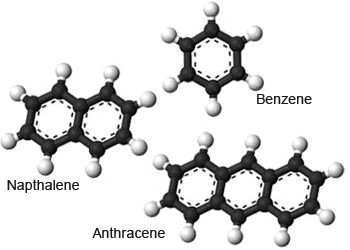
 Aromatic structures
Aromatic structures
Aromatic compounds are group of unsaturated hydrocarbons, which do not have straight–chain or branched
molecules–they have a ring structure. The systematic name for the aromatic hydrocarbons is arenes.
Benzene is the compound that forms the basis of the chemistry of all the aromatic hydrocarbons.
C6H6 has three double bonds contained within a flat hexagonal ring, unlike the double–bond electrons
in most other unsaturated hydrocarbons. However, the electrons of the double bonds in benzene are not fixed between any two carbon
atoms. Instead, these electrons are able to move freely around the ring. This is commonly represented by drawing a circle within
the ring, rather than the individual double bonds.
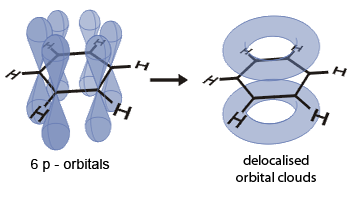 Delocalized structure of Benzene
Delocalized structure of Benzene
Benzene is a colorless liquid that is immiscible with water and has a characteristic odor. Its boiling point is 80°C and its melting point is 6°C. The benzene molecule has a symmetrical six–membered ring structure, which accounts for its relatively high melting and boiling points, as the rings pack tightly into a crystal lattice. Many important compounds contain benzene rings, for example, an alkyl group substituted for one or more of the hydrogen atoms around the carbon ring. The melting point of methyl benzene, −95°C, illustrates the affect of these alkyl side chains on the packing of the benzene molecules.
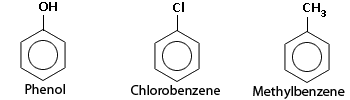 Hydrogen atom substituted Benzene rings.
Hydrogen atom substituted Benzene rings.
Many organic compounds contain one or more benzene rings in their structure. Because many of these compounds are fragrant, any organic molecule containing a benzene ring is classified as an aromatic compound (even if it is not particularly fragrant).
Most of the reactions of benzene produce other aromatic compounds. The hydrogen atoms in the benzene ring may be substituted by other atoms or groups to give compounds such as phenol, chlorobenzene or methyl benzene, shown in the figure. The addition reactions do not take place because of the stability of the molecule due to the delocalized electrons. Thus most of the reactions of benzene involve electrophilic substitution, the substitution of a hydrogen by an electrophile.