 In this method temperature of furnace is maintained just above the melting point of metal to be separated
which allows liquid of pure metal to be flown down without impurities.
In this method temperature of furnace is maintained just above the melting point of metal to be separated
which allows liquid of pure metal to be flown down without impurities.
Free metals obtained by various reduction processes may have several impurities, which may be other metals. For example while obtaining Na, K is a common metal that may be separated along with Na. Thus K is an impurity here. These impurities have to be removed by processes known as refining. Refining is nothing but purification of metals. There are various methods of refining; some of which we will discuss below:
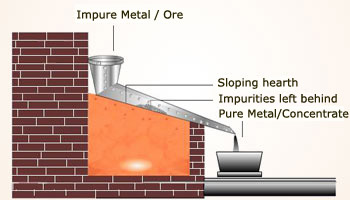
Liquation method:
In liquation method, metals with low melting points are refined. Metals like Sn, Pb, Bi have low melting points compared to the
impurities in the metal. A block of impure metal is placed on a sloping furnace. The temperature of the furnace is maintained
a bit above the melting point of the metal to be refined. The metal melts and flows off the slope, it is then collected and
cooled.
 Easily vaporizable metals are subjected to distillation. Metals when subjected to heating give vapors.
Vapors are then collected over and are cooled to retain the pure metal.
Easily vaporizable metals are subjected to distillation. Metals when subjected to heating give vapors.
Vapors are then collected over and are cooled to retain the pure metal.
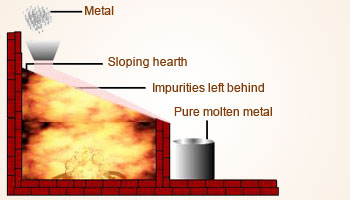
Distillation method:
Zn, Cd and Hg form vapors easily. They can be heated and distilled out from their impurities. Impure metal block is heated at a
temperature so that the metal atoms start to evaporate. The temperature is then held constant. The vapors are condensed and
separated out in a container called receiver. Non – volatile impurities are left behind in the distillation chamber.
 Electrolytic refining of metal
Purification of copper
Electrolytic refining of metal
Purification of copper
Oxidation method:
Sometimes impurities are able to get oxidized more easily than the metal itself. In this case oxidative method is used.
For example, if impurities are S, C, Si or P, they can get oxidized more easily than the metal itself. For example in case of
pig iron Fe, these non – metals are present as impurities. When air is passed over hot molten pig iron, these non –
metals get oxidized to CO2, SO2, P2O5 and can be removed easily.
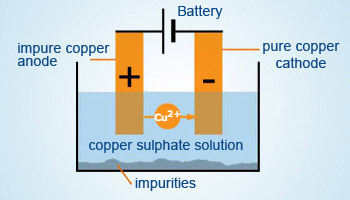
Electrolytic refining:
In this method electrolysis is used to refine metals. Metals like Cu, Zn, Sn, Pb, Ag, Au are refined by electrolysis method.
In an electrolytic cell, a block of impure metal is made into the anode, a thin strip of pure metal is made into a cathode and
an electrolyte is made out of a suitable metal – salt of the metal to be refined. When an electric current is passed
through the cell, ions from the anode enter the electrolyte. The same number of metal ions from the electrolyte gets deposited
on the cathode. This is a preferential deposition. Impurities remain in the electrolyte. Some of the impurities may be deposited
below the anode.