 Sodium is a soft silvery–white metal that can be readily cut with a knife.
Sodium is a soft silvery–white metal that can be readily cut with a knife.
 Sodium on flame test gives yellow color
Sodium on flame test gives yellow color
Sodium is an alkali IA group metal. Discovered by Humphry Davy in 1807 by the electrolysis of dried NaOH.
Sodium is a soft metal that can be readily cut with a knife and is a good conductor of electricity. It has a bright, silvery luster, on exposure to air it rapidly tarnishes, forming a white coating of sodium hydroxide and sodium carbonate. When sodium or its compounds are introduced into a flame,they burn with yellow color.
Sodium compounds are commercially used in very large scale in glass, paper, textiles and soap producing industries. These commercial important compounds include table salt(NaCl), caustic soda(NaOH), soda ash (Na2CO3), baking soda (NaHCO3), sodium nitrate(NaNO3), di – and tri –sodium phosphates, sodium thiosulfate (Na2S2O3.5H2O), and borax (Na2B4O7.10H2O).
 Sodium chloride from sea water
Sodium chloride from sea water
Preparation and properties of some important compounds of Sodium:
Sodium chloride
Common salt is a white crystalline solid, an ionic compound of sodium and chlorine. The main source of common salt (sodium chloride) is the sea water. Sodium chloride is also found as rock salt.
Commercial preparation of sodium chloride:
Common salt (or sodium chloride) is obtained on commercial scale from sea water(collected in small, rectangular or square lagoons) by evaporation in sunlight. After all the water has evaporated, crystals of common salt are left behind.
Sodium chloride is a deliquescent, i.e., it absorbs moisture from the atmosphere. This is due to the presence of small quantity of magnesium chloride (MgCl2) in it. Molten sodium chloride is a very good ionic conductor.
Uses of sodium chloride:
- Sodium chloride is used for manufacture of chemical compounds, such as caustic soda, soda ash (washing soda), baking soda, hydrochloric acid, chlorine gas, etc.
- It forms an essential ingredient in our food and used as a preservative for vegetables, meat and fish.
- It is also used in freezing mixtures producing low temperature.
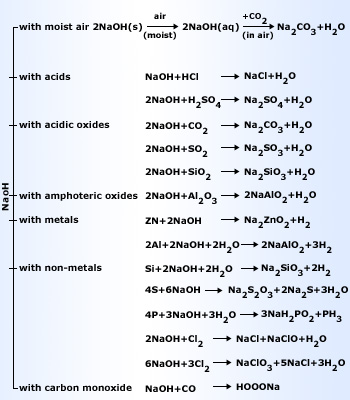 Chemical properties of sodium hydroxide
Chemical properties of sodium hydroxide
Sodium hydroxide
Sodium hydroxide is commonly called caustic soda because of its corrosive action on animal and vegetable tissues. Large quantity of sodium hydroxide is now–a–days prepared by electrolytic process.
Methods of Preparation of Sodium Hydroxide
Sodium hydroxide is prepared by Causticization process and Castner Kellner Process.
Causticization process depends on reaction between suspension of lime (Calcium hydroxide) and Sodium carbonate. This reaction is reversible.

In castner–kellner method NaOH is prepared by the electrolysis of aqueous solution of NaCl(Brine), it is a highly effective method. NaOH obtained in this method is highly pure.
Chemical properties of sodium hydroxide
Sodium hydroxide is a white deliquescent solid, is highly soluble in water with considerable amount of heat evolved. Aqueous solution of sodium hydroxide is strongly alkaline due to its complete dissociation into Na+ and OH–.
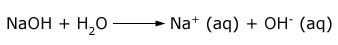
 Sodium hydroxide or caustic soda is widely used as a chemical base in several industries such as in the production of paper, textiles, drinking water, soaps and detergents.
Sodium hydroxide or caustic soda is widely used as a chemical base in several industries such as in the production of paper, textiles, drinking water, soaps and detergents.
Uses of Sodium Hydroxide
- In the manufacture of soap, paper, viscose rayon (artificial silk), organic dye stuffs, sodium metal and many other chemicals.
- Used in the refining of petroleum and vegetable oils, in the purification of bauxite for the extraction of aluminum.
- As a cleansing agent and in washing powder, for cleaning machines and metal sheets etc. It is too caustic (corrosive) to be used for washing clothes or hands.
- It is also used for mercerizing cotton, as a reagent in the laboratory, in reclaiming rubber and in the preparation of soda lime.
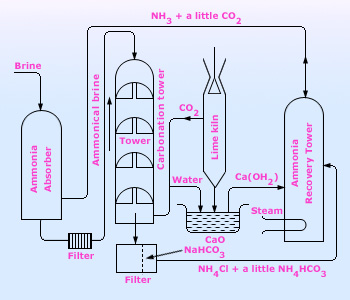 The plant used in the Solvay process for the manufacture of sodium carbonate
The plant used in the Solvay process for the manufacture of sodium carbonate
Sodium carbonate
Sodium carbonate exists as anhydrous salt (Na2CO3) and also as hydrated salt. The decahydrate (Na2CO3.10H2O) is known as washing soda. The anhydrous salt is called soda ash.
Preparation method
Sodium carbonate at industrial level is manufactured by the Solvay or Ammonia–soda process.
The raw materials for this process are common salt, ammonia and limestone (for supplying CO2 and quicklime). When carbon dioxide is passed into a concentrated solution of brine saturated with ammonia, ammonium bicarbonate is produced. The ammonium bicarbonate then reacts with common salt forming sodium bicarbonate.
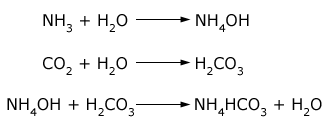
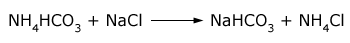
Sodium bicarbonate being slightly soluble (in presence of sodium ions) gets precipitated. The precipitated sodium bicarbonate is removed by filtration and changed into sodium carbonate by heating.
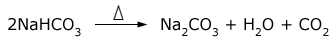
 Sodium carbonate used in detergent manufacture
Sodium carbonate used in detergent manufacture
Uses of Sodium Carbonate
- Used for the manufacture of glass.
- Used for washing purposes in laundries.
- Used for the manufacture of other sodium compounds like sodium silicate, sodium hydro–xide, borax, hypo etc.
- Used as a household cleansing agent.
- Used in paper and soap/detergent industries.
- Used for the softening of water.