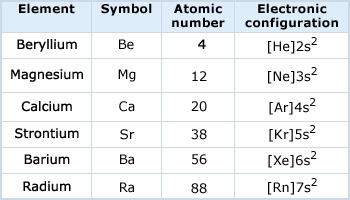
 Electronic configuration of IIA group elements
The outer shell electronic configuration of IIA group elements is ns2.
Electronic configuration of IIA group elements
The outer shell electronic configuration of IIA group elements is ns2.
The alkaline earth metals are the elements located in IIA Group of the periodic table. They are Beryllium(Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). They are placed in S – block in the periodic table as the outermost electrons of these elements are present in s–orbital.
The oxides of these metals form basic(alkaline) compounds when combined with water hence called the alkaline metals.

The alkaline earth metals are all silvery white solids, soft, and have relatively low densities, electron affinities and electronegativities. The melting points and boiling points are higher compared to alkali metals.
All the alkaline earth metals have two loosely bound electrons in their valence shell, they readily loose these electrons forming divalent cations to get stable electronic configuration(Inert gas configuration).
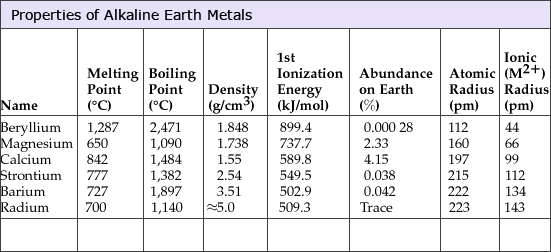
Physical properties of alkaline earth metals: Melting, Boiling points and ionization energies decreases down the group where as density and atomic radius increases due to addition of new shells down the group.
The alkaline earth metals react with the halogens to form ionic halides, such as calcium chloride (CaCl2), as well as reacts with oxygen forms oxides such as strontium oxide (SrO). Calcium, strontium and barium react with water to produce hydrogen gas and their respective hydroxides and also undergo transmetalation reactions to exchange ligands.
The alkaline earth metals are not found in their pure form in nature,they are obtained from their respective minerals in nature.
Occurrence of alkaline earth metals
Beryllium is not very abundant and it is difficult to extract. It is found in the minerals bertrandite
(Be4Si2O7(OH)2), beryl (Al2Be3Si6O18),
chrysoberyl (Al2BeO4) and phenakite (Be2SiO4). With a chromium–ion impurity,
beryl is known as emerald. If iron ions are present in beryl, the gemstone is blue–green known as aquamarine.
Beryllium is used as a lightweight component in military equipment and in the aerospace industry. It is used in high–speed aircraft, missiles, space vehicles, telescope mirrors and communication satellites. The most important use of beryllium is in radiation windows for X–ray tubes.
Magnesium is the 4th most abundant element in the earth's curst. It occurs in minerals such as dolomite(MgCO3.CaCO3), Epsomite (MgSO4.7H2O), magnesite (MgCO3), brucite(Mg(OH)2), carnallite(KCl.MgCl2.6H2O), talc (Mg3Si4O10(OH)2) and olivine( (Mg,Fe)2[SiO]4) are of commercial importance.
Magnesium is largely used in automobile and aircraft industry to manufacture parts of airplanes and automobiles.

Calcium is the 5th most abundant element in the Earth's crust. It occurs in the forms of carbonate, sulfate, fluoride, silicate and borate. calcium carbonate occurs in marble, chalk, limestone and calcite. Calcium sulfate (CaSO4) occurs in anhydrite and gypsum, calcium fluoride in fluorspar or fluorite (CaF2) and calcium phosphate occurs in Fluoroapatite [3(Ca3(PO4)2).CaF2]. Calcium also occurs in numerous silicates and alumino silicates. It occurs in almost all natural waters as calcium carbonates and calcium sulfate.
Calcium is essential for proper growth of the human body. Calcium compounds are also used on large scale for manufacturing construction materials such as cement.
 Strontium imparts a crimson color to fireworks
Strontium imparts a crimson color to fireworks
Strontium is the 15th most abundant element in the earth's crust. It occurs in minerals celestine (strontium sulfate, SrSO4) and strontianite (strontium carbonate, SrCO3). Strontium is used in atomic clocks, fireworks, toothpastes, producing glass (cathode ray tubes for color televisions) and also used in cancer therapy.
 Barium was first discovered in barite, it was found to radiate red light after heating.
Barium was first discovered in barite, it was found to radiate red light after heating.
Barium is the 14th most abundance element in the Earth's crust. It's main
sources are of barite also called as barytes or heavy spar, which is a barium sulfate mineral and witherite which is a barium
carbonate mineral.
Barium is used as a "flashed getter" in vacuum tubes,glassmaking, making superconductors, alloy of berium used in spark
plug wires.
 Radium used in self luminescence clock dial.
Radium used in self luminescence clock dial.
Radium is a radioactive element and is extremely scarce. It occurs in all ores that contain
uranium and in even in very small amounts in thorium minerals. It is a decay product of uranium and thorium.
Radium is used in making luminous paints, aircraft switches, clocks, nuclear panels and instrument dials which glow in the dark,
but it is not considered to be safe. Radium chloride is used to produce radon gas for cancer treatment.