The second law of thermodynamics states that for any process occurring in a closed system, the entropy increases for an irreversible system and remains constant for a reversible system, but never decreases.
The change in entropy of a system, referred as, ΔS depends only on the initial and final states of the system.
ΔS = S final – S initial
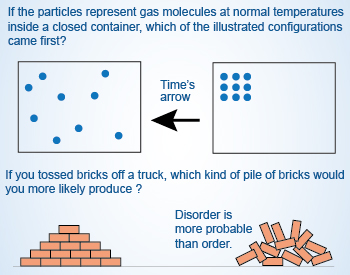
A positive value of ΔS indicates an increase in randomness or disorder.
The change in entropy of a system, ΔSsys , changes the entropy of the surroundings,
ΔS surr, because
the heat produced due to change in entropy of the system escapes into the surroundings.
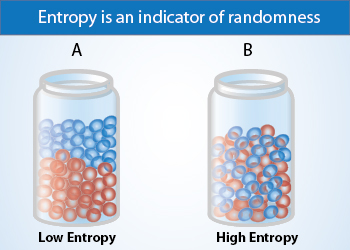 Entropy
A system which comprises of particles (atoms and molecules), arrange themselves in different ways, resulting in the increase in the entropy of the system, leading to the disorder of the system. The more disorder of a system, the larger its entropy.
Entropy
A system which comprises of particles (atoms and molecules), arrange themselves in different ways, resulting in the increase in the entropy of the system, leading to the disorder of the system. The more disorder of a system, the larger its entropy.
For example:
If a glass of hot liquid, is placed in a colder room, a potential exists (the entropy)
i.e., a flow of heat is spontaneously produced from the glass to the room until it
is minimized or maximized (at which point the temperatures are the same), this
leads to the change in entropy of the system, ΔSsys and the surroundings, ΔSsurr.
Since the universe is made up of the system and the surroundings, the entropy change in the universe, ΔSuniv for any process is the sum of the entropy change in the system, ΔSsys and the entropy change in the surroundings, ΔSsur.
ΔSuniv = ΔSsys + ΔSsurr
The conversion of energy which occur between the system, ΔSsys and the surroundings, ΔSsurr without any outside intervention are said to be spontaneous.