Conversion factors
Consider the balanced equation
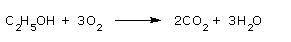
On a mole basis the following equivalences can be obtained:
1 mol C2H5OH = 3 mol O2
1 mol C2H5OH = 2 mol CO2
1 mol C2H5OH = 3 mol H2O
3 mol O2 = 2 mol CO2
3 mol O2 = 3 mol H2O
2 mol CO2 = 3 mol H2O
Once again, the equal sign does not represent mathematical equality but it should be read as "is equivalent to". These equalities apply only to the balanced equation from which they are derived.
Conversion factors from chemical formulae: The simplest ratio of atoms represents the empirical formula. Formulae for molecular compounds give the number and types of all the atoms that make up one molecule.
All chemical formulae represent the ratio of atoms within the formula. This fact allows the chemist to write the relationships between a formula as a whole and the individual atoms in that formula. For example, glucose with the formula C6H12O6.
1 molecule of C6H12O6 = 6 atoms of carbon
6 atoms of carbon = 12 atoms of hydrogen.
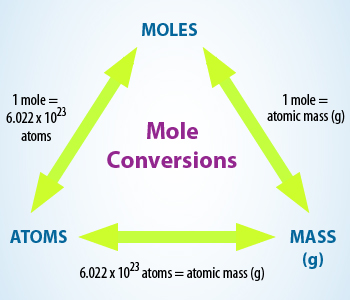 Chart showing conversions of moles,atoms and mass
Chart showing conversions of moles,atoms and mass
Percent composition:
To verify the purity of the sample for the use in laboratory experiment, we perform the experiment with mass spectrometer determining the masses of different elements and compare with the standard values. Thus it found exact the samples is supposed to be pure.

Where n is the number of moles of elements in 1 mole of the compound.
To calculate the percent composition of a component in a compound:
- Find the molar mass of the compound by adding up the masses of each atom in the compound using the periodic table or a molecular mass calculator.
- Calculate the mass due to the component in the compound for which you are solving by adding up the mass of these atoms.
- Divide the mass due to the component by the total molar mass of the compound and multiply by 100.
Example:Calculate the percentage of carbon in sucrose (C12H22O11)
Sol: Molar mass of the compound = 342.3 g / mol
Mass due to carbon:12 ( 12.01 ) = 144.12 g / mol
Percent composition of carbon = (144.12 g / mol / 342.3 g / mol) × 100 = 42.10 %
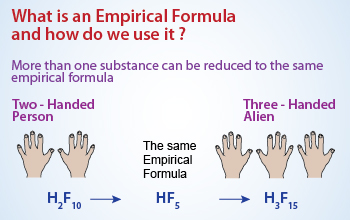
Empirical formulae:
Empirical formula is a formula which gives the simplest whole number ratio of atoms in a compound. The empirical formula tells the relative number of each type of atom, but it tells nothing about molecular structure. It tells the relative number of atoms of each element it contains. Thus, the formula H2SO4 indicates that sulphuric acid contains two H atoms. This ratio also applies on the molar level; thus 1 mol of H2SO4 contains 2 mol of H atoms and 1 mol of S atom and 4 mol of O atoms. Conversely, the ratio of the number of moles of each element in a compound gives the subscripts in a compound's empirical formula of chemical substances.
 Empirical Formula
Empirical formula is the simplest whole number ratio of all the elements present in the compound.
Empirical Formula
Empirical formula is the simplest whole number ratio of all the elements present in the compound.
Molecular formulae:
Molecular formulae are the chemical formulae that indicate the actual numbers and types of atoms in a molecule.
Molecular formulae can be obtained from empirical formula if we know the molecular weight of the compound. Molecular formulae provide greater information about molecules than do empirical formulae.
The subscripts in the molecular formula of a substance are always a whole–number multiple of the corresponding subscripts in its empirical formula. The multiple is found by comparing the formula weight of the empirical formula with the molecular weight.
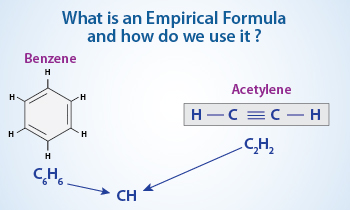
The empirical formula for water, H2O, is also the correct molecular formula for water. The molecular formula for water specifies that two hydrogen atoms are bound to a single oxygen atom. Compounds may also share an empirical formula but dramatically differ in their molecular formula.
Acetylene and benzene both have an empirical formula of CH. Acetylene has a molecular formula C2H2 while benzene, has a molecular formula of C6H6. In this regard, a molecule was considered to be a unit or the smallest particle of a compound that retained the chemical properties of the substance.