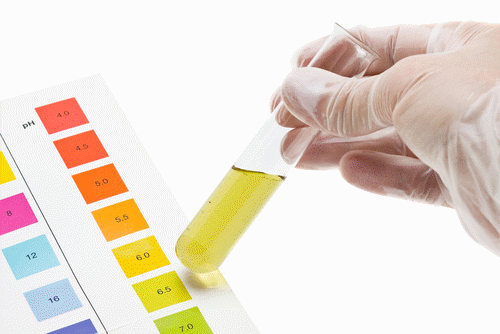
 Acids produce protons or the H+ ion while bases accept protons or generate OH−.
Acids produce protons or the H+ ion while bases accept protons or generate OH−.
Acids : An acid is defined as a substance that gives off protons while base is a substance that accepts protons.
Thus, an acid is a proton (H+) donor and a base is a proton acceptor.
What would cause an aqueous solution to have an imbalance in its H+ and OH− concentrations?
When the substances called acids dissolve in water, they donate additional H+ to the solution.
An acid, according to the definition often used by biologists, is a substance that increases the hydrogen ion concentration
of a solution. For example, when hydrochloric acid (HCl) is added to water, hydrogen ions dissociate from chloride ions:
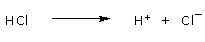
This additional source of H+(dissociation of water is the other source) results in the solution having more
H+ than OH− . Such a solution is known as an acidic solution.
Bases: A substance that reduces the hydrogen ion concentration of a solution is called a base.
Some bases reduce the H+ concentration directly by accepting hydrogen ions.
Ammonia (NH3), for instance, acts as a base when the unshared electron pair in nitrogen's valence shell
attracts a hydrogen ion from the solution, resulting in an ammonium ion (NH4+):
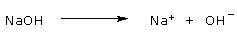
Solutions with a higher concentration of OH− than H+ are known as basic solutions.
 The pH levels in the blood are required to stay neutral, which is at a level of 7.
The pH levels in the blood are required to stay neutral, which is at a level of 7.
Significance: Acids and bases function to balance the pH levels in the body. Acids and bases are found in foods, the environment and in chemicals including pharmaceuticals. The pH levels in the blood are required to stay neutral, which is at a level of 7. When a dieter eats acidic foods, the body uses a buffering system to neutralize the positive ions released from the acids. Conversely, bases are also controlled to keep the body from becoming too alkaline.