 Covalent bonds result from sharing of electrons.
Covalent bond is a type of electrical attraction in which atoms are held together by their mutual attraction
for shared electrons is known as a covalent bond (co–signifies sharing and valent refers to the valence electrons being shared).
Covalent bonds result from sharing of electrons.
Covalent bond is a type of electrical attraction in which atoms are held together by their mutual attraction
for shared electrons is known as a covalent bond (co–signifies sharing and valent refers to the valence electrons being shared).
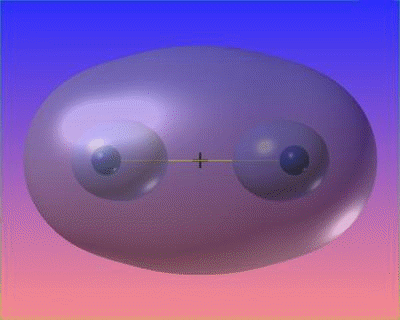
Covalent bonds result from a sharing of electrons. Imagine two little girls playing with a doll – each one is tugging at the doll.
The force that keeps the children together is their mutual attraction to the toy they share. In a similar fashion, two or three atoms can be held together by their mutual attraction for electrons they share. This type of electrical attraction in which atoms are held together by their mutual attraction for shared electrons is known as a covalent bond (co–signifies sharing and valent refers to the valence electrons being shared). Thus atomic bond formed by sharing of electrons is called a covalent bond. Compounds that are formed due to covalent bonding of atoms are called covalent compounds.
Covalent bonds are generally formed between non–metals. There are many atoms where the electronic configuration of the last orbits or shells is not as simple as Na or Cl. For example, in an Oxygen (O) atom the electronic configuration is: 2 electrons in the K shell and 6 electrons in the L shell. It would rather borrow two electrons from some other atom to complete its last shell than give up its six electrons. Hydrogen (H) atom has only one electron and needs to borrow only one extra electron to complete its first shell. Two atoms of H and one atom of O fulfill each others needs by sharing electrons in their outermost orbits and form H2O or a water molecule. In an H2O molecule, the electrons are not totally given up, as in the case of Na+− Cl−, but are shared by each of the neighboring atoms.